Evolution of Salmonella within Hosts
- PMID: 29954653
- PMCID: PMC6249985
- DOI: 10.1016/j.tim.2018.06.001
Evolution of Salmonella within Hosts
Abstract
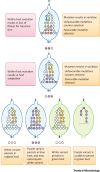
Within-host evolution has resulted in thousands of variants of Salmonella that exhibit remarkable diversity in host range and disease outcome, from broad host range to exquisite host restriction, causing gastroenteritis to disseminated disease such as typhoid fever. Within-host evolution is a continuing process driven by genomic variation that occurs during each infection, potentiating adaptation to a new niche resulting from changes in animal husbandry, the use of antimicrobials, and emergence of immune compromised populations. We discuss key advances in our understanding of the evolution of Salmonella within the host, inferred from (i) the process of host adaptation of Salmonella pathovars in the past, and (ii) direct observation of the generation of variation and selection of beneficial traits during single infections.
Keywords: Salmonella; adaptation; evolution; pathogenesis.
Copyright © 2018 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures


References
-
- Kingsley R.A., Baumler A.J. Host adaptation and the emergence of infectious disease: the Salmonella paradigm. Mol. Microbiol. 2000;36:1006–1014. - PubMed
-
- Diard M. Stabilization of cooperative virulence by the expression of an avirulent phenotype. Nature. 2013;494:353–356. - PubMed
-
- Ackermann M. Self-destructive cooperation mediated by phenotypic noise. Nature. 2008;454:987–990. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

