Exposure-adjusted adverse events comparing blinatumomab with chemotherapy in advanced acute lymphoblastic leukemia
- PMID: 29954814
- PMCID: PMC6039663
- DOI: 10.1182/bloodadvances.2018019034
Exposure-adjusted adverse events comparing blinatumomab with chemotherapy in advanced acute lymphoblastic leukemia
Abstract
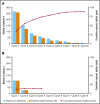
In the phase 3 TOWER study, blinatumomab demonstrated an overall survival benefit over standard-of-care chemotherapy (SOC) in adults with relapsed or refractory (r/r) Philadelphia chromosome-negative (Ph-) B-precursor acute lymphoblastic leukemia (ALL). Nearly all patients in both treatment arms experienced an adverse event (AE), and the incidence rate of serious AEs was higher for blinatumomab. However, as treatment exposure differed between the 2 arms, we conducted an exploratory safety analysis comparing exposure-adjusted event rates (EAERs) of blinatumomab vs SOC. Analyses were conducted for all patients who received therapy (safety population). Patients received a median (range) of 2 cycles (1-9) of blinatumomab (N = 267) vs 1 cycle (1-4) of SOC (N = 109). Grade ≥3 AE rates were generally higher in cycle 1 of blinatumomab than in cycle 2 (76% vs 37%). After adjusting for time on treatment, EAERs of grade ≥3 were significantly lower for blinatumomab vs SOC overall (10.73 vs 45.27 events per patient-year; P < .001) and for events of clinical interest, including infections (1.63 vs 6.49 events per patient-year; P < .001), cytopenias (3.64 vs 20.07 events per patient-year; P < .001), and neurologic events (0.38 vs 0.95 events per patient-year; P = .008). The EAER of grade ≥3 cytokine-release syndrome was higher for blinatumomab than for SOC (0.16 vs 0 events per patient-year; P = .038). These data further support the role of blinatumomab as an efficacious and well-tolerated treatment option for patients with r/r Ph- ALL. This trial was registered at www.clinicaltrials.gov as #NCT02013167.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: A.S.S. received consulting fees and is a member of the speaker’s bureau for Amgen Inc. R.A.L received consulting fees from Amgen Inc. A.C.S. received consulting fees from Amgen Inc. W.S. received honoraria from Amgen Inc. E.L.-M. received consulting fees from Amgen Inc, Roche, and Janssen-Cilag. Q.T. is an employee of Amgen Inc. Z.Z. and W.K. are employees of and hold stock in Amgen Inc. M.S.T. received research grants from Amgen Inc, Gilead, Macrogenics, Roche, and Regeneron, as well as consulting fees from Amgen Inc, Roche, and Regeneron.
Figures

References
-
- Goldstone AH, Richards SM, Lazarus HM, et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993). Blood. 2008;111(4):1827-1833. - PubMed
-
- Bassan R, Hoelzer D. Modern therapy of acute lymphoblastic leukemia. J Clin Oncol. 2011;29(5):532-543. - PubMed
-
- Nishiwaki S, Inamoto Y, Sakamaki H, et al. Allogeneic stem cell transplantation for adult Philadelphia chromosome-negative acute lymphocytic leukemia: comparable survival rates but different risk factors between related and unrelated transplantation in first complete remission. Blood. 2010;116(20):4368-4375. - PubMed
-
- Fielding AK, Richards SM, Chopra R, et al. ; Eastern Cooperative Oncology Group. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood. 2007;109(3):944-950. - PubMed
-
- Tavernier E, Boiron JM, Huguet F, et al. ; Australasian Leukaemia and Lymphoma Group. Outcome of treatment after first relapse in adults with acute lymphoblastic leukemia initially treated by the LALA-94 trial. Leukemia. 2007;21(9):1907-1914. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

