Lower DHEA-S levels predict disease and worse outcomes in post-menopausal women with idiopathic, connective tissue disease- and congenital heart disease-associated pulmonary arterial hypertension
- PMID: 29954925
- PMCID: PMC6469347
- DOI: 10.1183/13993003.00467-2018
Lower DHEA-S levels predict disease and worse outcomes in post-menopausal women with idiopathic, connective tissue disease- and congenital heart disease-associated pulmonary arterial hypertension
Abstract
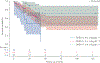
High oestradiol (E2) and low dehydroepiandrosterone-sulfate (DHEA-S) levels are risk factors for pulmonary arterial hypertension (PAH) in men, but whether sex hormones are related to PAH in women is unknown.Post-menopausal women aged ≥55 years with PAH were matched by age and body mass index to women without cardiovascular disease. Plasma sex hormone levels were measured by immunoassay.Lower levels of DHEA-S (p<0.001) and higher levels of E2 (p=0.02) were associated with PAH. In PAH cases (n=112), lower DHEA-S levels were associated with worse haemodynamics (all p<0.01) and more right ventricular dilatation and dysfunction (both p=0.001). Lower DHEA-S levels were associated with shorter 6-min walking distance (6MWD) (p=0.01) and worse functional class (p=0.004). Each Ln(1 µg·dL-1) decrease in DHEA-S was associated with a doubling in the risk of death (hazard ratio 2.0, 95% CI 1.5-2.7; p<0.001). Higher levels of E2 were associated with shorter 6MWD (p=0.03) and worse functional class (p=0.01).High E2 and low DHEA-S levels are associated with the risk and severity of PAH in post-menopausal women. Hormonal modulation should be studied as a treatment strategy in PAH.
Copyright ©ERS 2018.
Conflict of interest statement
Conflict of interest: S.M. Kawut reports grants (for research and CME) from Actelion, United Therapeutics, Gilead, Lung Biotech, Bayer and the Cardiovascular Medical Research and Education Fund, and personal fees (for travel) from the American Thoracic Society, outside the submitted work. Conflict of interest: J.R. Klinger reports grants from Actelion, United Therapeutics, Gilead, Eiger and Bayer, outside the submitted work. Conflict of interest: H.I. Palevsky reports personal fees from Actelion, Janssen, Bayer, GSK and United Therapeutics, outside the submitted work. Conflict of interest: I.R. Preston reports grants and personal fees from Actelion, grants from Bayer and Gilead, and personal fees from Arena, United Therapeutics and Pfizer, outside the submitted work. Conflict of interest: A. Smith reports grants from Actelion, Gilead and United Therapeutics, outside the submitted work. Conflict of interest: M. Whittenhall reports personal fees from Actelion, Bayer, Gilead and United Therapeutics, outside the submitted work. Conflict of interest: C.E. Ventetuolo reports personal fees from Bayer, Acceleron and United Therapeutics and grants from the CHEST Foundation (Actelion sponsored) and Eiger paid to her institution, outside the submitted work. Conflict of interest: C. Archer-Chicko reports personal fees from Actelion, outside the submitted work.
Figures
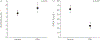
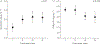

Comment in
-
ACE2 as therapy for pulmonary arterial hypertension: the good outweighs the bad.Eur Respir J. 2018 Jun 21;51(6):1800848. doi: 10.1183/13993003.00848-2018. Print 2018 Jun. Eur Respir J. 2018. PMID: 29929959 No abstract available.
-
Beyond oestrogens: towards a broader evaluation of the hormone profile in pulmonary arterial hypertension.Eur Respir J. 2018 Jun 28;51(6):1801058. doi: 10.1183/13993003.01058-2018. Print 2018 Jun. Eur Respir J. 2018. PMID: 29954927 Free PMC article. No abstract available.
References
-
- Ling Y, Johnson MK, Kiely DG, et al. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am J Respir Crit Care Med 2012; 186: 790–796. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HHSN268201500003C/HL/NHLBI NIH HHS/United States
- R01 HL077612/HL/NHLBI NIH HHS/United States
- N01 HC095167/HL/NHLBI NIH HHS/United States
- N01 HC095159/HL/NHLBI NIH HHS/United States
- P20 GM103652/GM/NIGMS NIH HHS/United States
- UL1 TR001420/TR/NCATS NIH HHS/United States
- N01 HC095163/HL/NHLBI NIH HHS/United States
- UL1 TR000040/TR/NCATS NIH HHS/United States
- N01 HC095166/HL/NHLBI NIH HHS/United States
- N01 HC095162/HL/NHLBI NIH HHS/United States
- N01 HC095160/HL/NHLBI NIH HHS/United States
- L30 HL115758/HL/NHLBI NIH HHS/United States
- N01 HC095161/HL/NHLBI NIH HHS/United States
- N01 HC095168/HL/NHLBI NIH HHS/United States
- HHSN263201500003I/NH/NIH HHS/United States
- UL1 TR001079/TR/NCATS NIH HHS/United States
- N01 HC095169/HL/NHLBI NIH HHS/United States
- R01 HL086719/HL/NHLBI NIH HHS/United States
- HHSN268201500003I/HL/NHLBI NIH HHS/United States
- N01 HC095165/HL/NHLBI NIH HHS/United States
- K24 HL103844/HL/NHLBI NIH HHS/United States
- N01 HC095164/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
