A phase II/III randomized, multicenter trial of prednisone/sirolimus versus prednisone/ sirolimus/calcineurin inhibitor for the treatment of chronic graft- versus-host disease: BMT CTN 0801
- PMID: 29954931
- PMCID: PMC6278959
- DOI: 10.3324/haematol.2018.195123
A phase II/III randomized, multicenter trial of prednisone/sirolimus versus prednisone/ sirolimus/calcineurin inhibitor for the treatment of chronic graft- versus-host disease: BMT CTN 0801
Abstract
Initial therapy of chronic graft-versus-host disease is prednisone ± a calcineurin-inhibitor, but most patients respond inadequately. In a randomized, adaptive, phase II/III, multicenter trial we studied whether prednisone/sirolimus or prednisone/sirolimus/photopheresis was more effective than prednisone/sirolimus/calcineurin-inhibitor for treating chronic graft-versus-host disease in treatment-naïve or early inadequate responders. Primary endpoints of this study were proportions of subjects alive without relapse or secondary therapy with 6-month complete or partial response in phase II, or with 2-year complete response in phase III. The prednisone/sirolimus/photopheresis arm closed prematurely because of slow accrual and the remaining two-drug versus three-drug study ended in phase II due to statistical futility with 138 evaluable subjects. The two-drug and three-drug arms did not differ in rates of 6-month complete or partial response (48.6% versus 50.0%, P=0.87), or 2-year complete response (14.7% versus 15.5%, P=0.90). Serum creatinine values >1.5 times baseline were less frequent in the calcineurin-inhibitor-free arm at 2 months (1.5% versus 11.7%, P=0.025) and 6 months (7.8% versus 24.0%, P=0.016). Higher adjusted Short Form-36 Physical Component Summary and Physical Functioning scores were seen in the two-drug arm at both 2 months (P=0.02 and P=0.04, respectively) and 6 months (P=0.007 and P=0.001, respectively). Failure-free survival and overall survival rates at 2 years were similar for patients in the the two-drug and three-drug arms (48.6% versus 46.2%, P=0.78; 81.5% versus 74%, P=0.28). Based on similar long-term outcomes, prednisone/sirolimus is a therapeutic alternative to prednisone/sirolimus/calcineurin-inhibitor for chronic graft-versus-host disease, being easier to administer and better tolerated. Clinicaltrials.gov identifier: NCT01106833.
Copyright© 2018 Ferrata Storti Foundation.
Figures
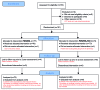

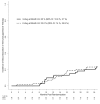


References
-
- Socie G, Stone JV, Wingard JR, et al. Long-term survival and late deaths after allogeneic bone marrow transplantation. Late Effects Working Committee of the International Bone Marrow Transplant Registry. N Engl J Med. 1999;341(1):14–21. - PubMed
-
- Sullivan KM, Witherspoon RP, Storb R, et al. Prednisone and azathioprine compared with prednisone and placebo for treatment of chronic graft-versus-host disease: prognostic influence of prolonged thrombocytopenia after allogeneic marrow transplantation. Blood. 1988;72(2):546–554. - PubMed
-
- Koc S, Leisenring W, Flowers ME, et al. Therapy for chronic graft-versus-host disease: a randomized trial comparing cyclosporine plus prednisone versus prednisone alone. Blood. 2002;100(1):48–51. - PubMed
-
- Arora M, Wagner JE, Davies SM, et al. Randomized clinical trial of thalidomide, cyclosporine, and prednisone versus cyclosporine and prednisone as initial therapy for chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2001;7(5):265–273. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10 HL069294/HL/NHLBI NIH HHS/United States
- R01 HL129061/HL/NHLBI NIH HHS/United States
- P01 CA015396/CA/NCI NIH HHS/United States
- UG1 HL069249/HL/NHLBI NIH HHS/United States
- U10 HL069291/HL/NHLBI NIH HHS/United States
- UG1 HL069278/HL/NHLBI NIH HHS/United States
- UG1 HL069246/HL/NHLBI NIH HHS/United States
- P01 CA049605/CA/NCI NIH HHS/United States
- UG1 HL069301/HL/NHLBI NIH HHS/United States
- UG1 HL069290/HL/NHLBI NIH HHS/United States
- U24 CA076518/CA/NCI NIH HHS/United States
- U24 HL138660/HL/NHLBI NIH HHS/United States
- UG1 HL069254/HL/NHLBI NIH HHS/United States
- UG1 HL069291/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

