A method for single-neuron chronic recording from the retina in awake mice
- PMID: 29954976
- PMCID: PMC6047945
- DOI: 10.1126/science.aas9160
A method for single-neuron chronic recording from the retina in awake mice
Abstract
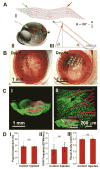
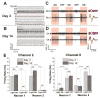
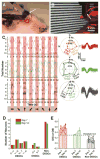
The retina, which processes visual information and sends it to the brain, is an excellent model for studying neural circuitry. It has been probed extensively ex vivo but has been refractory to chronic in vivo electrophysiology. We report a nonsurgical method to achieve chronically stable in vivo recordings from single retinal ganglion cells (RGCs) in awake mice. We developed a noncoaxial intravitreal injection scheme in which injected mesh electronics unrolls inside the eye and conformally coats the highly curved retina without compromising normal eye functions. The method allows 16-channel recordings from multiple types of RGCs with stable responses to visual stimuli for at least 2 weeks, and reveals circadian rhythms in RGC responses over multiple day/night cycles.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

