7B2 chaperone knockout in APP model mice results in reduced plaque burden
- PMID: 29955078
- PMCID: PMC6023903
- DOI: 10.1038/s41598-018-28031-7
7B2 chaperone knockout in APP model mice results in reduced plaque burden
Abstract
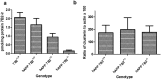
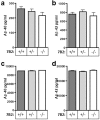
Impairment of neuronal proteostasis is a hallmark of Alzheimer's and other neurodegenerative diseases. However, the underlying molecular mechanisms leading to pathogenic protein aggregation, and the role of secretory chaperone proteins in this process, are poorly understood. We have previously shown that the neural-and endocrine-specific secretory chaperone 7B2 potently blocks in vitro fibrillation of Aβ42. To determine whether 7B2 can function as a chaperone in vivo, we measured plaque formation and performed behavioral assays in 7B2-deficient mice in an hAPPswe/PS1dE9 Alzheimer's model mouse background. Surprisingly, immunocytochemical analysis of cortical levels of thioflavin S- and Aβ-reactive plaques showed that APP mice with a partial or complete lack of 7B2 expression exhibited a significantly lower number and burden of thioflavin S-reactive, as well as Aβ-immunoreactive, plaques. However, 7B2 knockout did not affect total brain levels of either soluble or insoluble Aβ. While hAPP model mice performed poorly in the Morris water maze, their brain 7B2 levels did not impact performance. Since 7B2 loss reduced amyloid plaque burden, we conclude that brain 7B2 can impact Aβ disposition in a manner that facilitates plaque formation. These results are reminiscent of prior findings in hAPP model mice lacking the ubiquitous secretory chaperone clusterin.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

