miR-137 is a tumor suppressor in endometrial cancer and is repressed by DNA hypermethylation
- PMID: 29955087
- PMCID: PMC6214735
- DOI: 10.1038/s41374-018-0092-x
miR-137 is a tumor suppressor in endometrial cancer and is repressed by DNA hypermethylation
Abstract
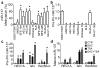
Endometrial cancer is the most common gynecological cancer in the United States. We wanted to identify epigenetic aberrations involving microRNAs (miRNAs), whose genes become hypermethylated in endometrial primary tumors. By integrating known miRNA sequences from the miRNA database (miRBase) with DNA methylation data from methyl-CpG-capture sequencing, we identified 111 differentially methylated regions (DMRs) associated with CpG islands (CGIs) and miRNAs. Among them, 22 DMRs related to 29 miRNAs and within 8 kb of CGIs were hypermethylated in endometrial tumors but not in normal endometrium. miR-137 was further validated in additional endometrial primary tumors. Hypermethylation of miR-137 was found in both endometrioid and serous endometrial cancer (P < 0.01), and it led to the loss of miR-137 expression. Treating hypermethylated endometrial cancer cells with epigenetic inhibitors reactivated miR-137. Moreover, genetic overexpression of miR-137 suppressed cancer cell proliferation and colony formation in vitro. When transfected cancer cells were implanted into nude mice, the cells that overexpressed miR-137 grew more slowly and formed smaller tumors (P < 0.05) than vector transfectants. Histologically, xenograft tumors from cancer cells expressing miR-137 were less proliferative (P < 0.05), partly due to inhibition of EZH2 and LSD1 expression (P < 0.01) in both the transfected cancer cells and tumors. Reporter assays indicated that miR-137 targets EZH2 and LSD1. These results suggest that miR-137 is a tumor suppressor that is repressed in endometrial cancer because the promoter of its gene becomes hypermethylated.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

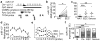


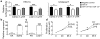
Similar articles
-
miR-152 is a tumor suppressor microRNA that is silenced by DNA hypermethylation in endometrial cancer.Cancer Res. 2011 Oct 15;71(20):6450-62. doi: 10.1158/0008-5472.CAN-11-0364. Epub 2011 Aug 25. Cancer Res. 2011. PMID: 21868754
-
Hypermethylation of miR-203 in endometrial carcinomas.Gynecol Oncol. 2014 May;133(2):340-5. doi: 10.1016/j.ygyno.2014.02.009. Epub 2014 Feb 14. Gynecol Oncol. 2014. PMID: 24530564 Free PMC article.
-
Epigenetic repression of microRNA-129-2 leads to overexpression of SOX4 oncogene in endometrial cancer.Cancer Res. 2009 Dec 1;69(23):9038-46. doi: 10.1158/0008-5472.CAN-09-1499. Epub 2009 Nov 3. Cancer Res. 2009. PMID: 19887623 Free PMC article.
-
EZH2 inhibition suppresses endometrial cancer progression via miR-361/Twist axis.Oncotarget. 2017 Feb 21;8(8):13509-13520. doi: 10.18632/oncotarget.14586. Oncotarget. 2017. PMID: 28088786 Free PMC article.
-
Carcinogenic mechanisms of endometrial cancer: involvement of genetics and epigenetics.J Obstet Gynaecol Res. 2014 Aug;40(8):1957-67. doi: 10.1111/jog.12442. J Obstet Gynaecol Res. 2014. PMID: 25131761 Review.
Cited by
-
MicroRNA as Epigenetic Modifiers in Endometrial Cancer: A Systematic Review.Cancers (Basel). 2021 Mar 6;13(5):1137. doi: 10.3390/cancers13051137. Cancers (Basel). 2021. PMID: 33800944 Free PMC article. Review.
-
Non-Coding RNAs as Prognostic Markers for Endometrial Cancer.Int J Mol Sci. 2021 Mar 19;22(6):3151. doi: 10.3390/ijms22063151. Int J Mol Sci. 2021. PMID: 33808791 Free PMC article. Review.
-
Mitochondrial Genetic and Epigenetic Regulations in Cancer: Therapeutic Potential.Int J Mol Sci. 2022 Jul 18;23(14):7897. doi: 10.3390/ijms23147897. Int J Mol Sci. 2022. PMID: 35887244 Free PMC article. Review.
-
The role of HOTAIR/miR-152-3p/LIN28B in regulating the progression of endometrial squamous carcinoma.Arch Med Sci. 2019 Nov 7;17(2):434-448. doi: 10.5114/aoms.2019.89632. eCollection 2021. Arch Med Sci. 2019. PMID: 33747279 Free PMC article.
-
MicroRNA-873 inhibits the proliferation and invasion of endometrial cancer cells by directly targeting hepatoma-derived growth factor.Exp Ther Med. 2019 Aug;18(2):1291-1298. doi: 10.3892/etm.2019.7713. Epub 2019 Jun 26. Exp Ther Med. 2019. Retraction in: Exp Ther Med. 2022 Jun 27;24(2):538. doi: 10.3892/etm.2022.11475. PMID: 31363373 Free PMC article. Retracted.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

