Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: pooled analyses after long-term follow-up in KEYNOTE-012
- PMID: 29955135
- PMCID: PMC6048158
- DOI: 10.1038/s41416-018-0131-9
Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: pooled analyses after long-term follow-up in KEYNOTE-012
Abstract
Background: Second-line treatment options for advanced head and neck squamous cell carcinoma (HNSCC) are limited. The phase Ib KEYNOTE-012 study evaluated the safety and the efficacy of pembrolizumab for the treatment of HNSCC after long-term follow-up.
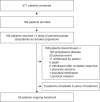
Methods: Multi-centre, non-randomised trial included two HNSCC cohorts (initial and expansion) in which 192 patients were eligible. Patients received pembrolizumab 10 mg/kg every 2 weeks (initial cohort; N = 60) or 200 mg every 3 weeks (expansion cohort; N = 132). Co-primary endpoints were safety and overall response rate (ORR; RECIST v1.1; central imaging vendor review).
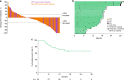
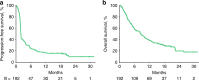
Results: Median follow-up was 9 months (range, 0.2-32). Treatment-related adverse events (AEs) of any grade and grade 3/4 occurred in 123 (64%) and 24 (13%) patients, respectively. No deaths were attributed to treatment-related AEs. ORR was 18% (34/192; 95% CI, 13-24%). Median response duration was not reached (range, 2+ to 30+ months); 85% of responses lasted ≥6 months. Overall survival at 12 months was 38%.
Conclusions: Some patients received 2 years of treatment and the responses were ongoing for more than 30 months; the durable anti-tumour activity and tolerable safety profile, observed with long-term follow-up, support the use of pembrolizumab as a treatment for recurrent/metastatic HNSCC.
Conflict of interest statement
T.Y.S., J.W., B.B., and H.K. have received research funding from Merck & Co., Inc. M.T. has received personal fees from Merck Sharp & Dohme. D.A.-G., A.R., K.P., and J.C. are employees Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. L.Q.C. has served as an advisor for and has received research from Merck & Co., Inc. R.H. has received research funding from Merck & Co., Inc., Bristol-Myers Squibb, Pfizer, and Astra Zeneca, and has served as a consultant for Merck & Co., Inc., Bristol-Myers Squibb, Pfizer, Celgene, Astra Zeneca, and Eisai. The remaining authors declare no competing interests.
Figures
Comment in
-
Immunotherapy for head and neck cancer: where are we now and where are we going?Ann Transl Med. 2019 Jul;7(Suppl 3):S75. doi: 10.21037/atm.2019.03.58. Ann Transl Med. 2019. PMID: 31576284 Free PMC article. No abstract available.
References
-
- National Comprehensive Cancer Network. NCCN clinical practice guidelines (NCCN Guidelines®) in oncology: head and neck cancers. Version 1.2018. https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf. Accessed 19 Apr 2018 (2018).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

