An altered CD8+ T cell epitope of insulin prevents type 1 diabetes in humanized NOD mice
- PMID: 29955175
- PMCID: PMC6804845
- DOI: 10.1038/s41423-018-0058-3
An altered CD8+ T cell epitope of insulin prevents type 1 diabetes in humanized NOD mice
Abstract
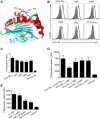
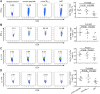
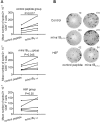
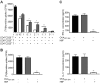
Autoreactive CD8+ T cells, which play an indispensable role in β cell destruction, represent an emerging target for the prevention of type 1 diabetes (T1D). Altered peptide ligands (APLs) can efficiently induce antigen-specific T cells anergy, apoptosis or shifts in the immune response. Here, we found that HLA-A*0201-restricted CD8+ T cell responses against a primary β-cell autoantigen insulin epitope InsB15-14 were present in both NOD.β2mnull.HHD NOD mice and T1D patients. We generated several APL candidates for InsB15-14 by residue substitution at the p6 position. Only H6F exhibited an inhibitory effect on mInsB15-14-specific CD8+ T cell responses in vitro. H6F treatment significantly reduced the T1D incidence, which was accompanied by diminished autoreactive CD8+ T cell responses to mInsB15-14, inhibited infiltration of CD8+ and CD4+ T cells in the pancreas and reduced pro-inflammatory cytokine production in pancreatic and splenic T cells in NOD.β2mnull.HHD mice. Mechanistically, H6F treatment significantly augmented a tiny portion of CD8+CD25+Foxp3+ T cells in the spleen and especially in the pancreas. This subset exhibited typical Treg phenotypes and required peptide-specific restimulation to exert immunosuppressive activity. Therefore, this APL H6F may be a promising candidate with potential clinical application value for antigen-specific prevention of T1D.
Keywords: Altered peptide ligand; CD8+CD25+Foxp3+ regulatory T cells; InsB15–14; NOD.β2m null .HHD mice; Type 1 diabetes.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
A Single L/D-Substitution at Q4 of the mInsA2-10 Epitope Prevents Type 1 Diabetes in Humanized NOD Mice.Front Immunol. 2021 Aug 30;12:713276. doi: 10.3389/fimmu.2021.713276. eCollection 2021. Front Immunol. 2021. PMID: 34526989 Free PMC article.
-
Prevention of "Humanized" diabetogenic CD8 T-cell responses in HLA-transgenic NOD mice by a multipeptide coupled-cell approach.Diabetes. 2011 Apr;60(4):1229-36. doi: 10.2337/db10-1523. Epub 2011 Feb 23. Diabetes. 2011. PMID: 21346176 Free PMC article.
-
Identification of autoreactive CD8+ T cell responses targeting chromogranin A in humanized NOD mice and type 1 diabetes patients.Clin Immunol. 2015 Jul;159(1):63-71. doi: 10.1016/j.clim.2015.04.017. Epub 2015 May 6. Clin Immunol. 2015. PMID: 25958206
-
Control of type 1 autoimmune diabetes by naturally occurring CD4+CD25+ regulatory T lymphocytes in neonatal NOD mice.Ann N Y Acad Sci. 2005 Jun;1051:72-87. doi: 10.1196/annals.1361.048. Ann N Y Acad Sci. 2005. PMID: 16126946 Review.
-
Bridging Mice to Men: Using HLA Transgenic Mice to Enhance the Future Prediction and Prevention of Autoimmune Type 1 Diabetes in Humans.Methods Mol Biol. 2016;1438:137-51. doi: 10.1007/978-1-4939-3661-8_9. Methods Mol Biol. 2016. PMID: 27150089 Review.
Cited by
-
Analysis of frequency changes in CD8+ regulatory T cell subsets in peripheral blood of individuals with type 1 diabetes.Diabetol Metab Syndr. 2024 Dec 19;16(1):305. doi: 10.1186/s13098-024-01549-9. Diabetol Metab Syndr. 2024. PMID: 39695694 Free PMC article.
-
Preclinical Models to Evaluate the Human Response to Autoantigen and Antigen-Specific Immunotherapy in Human Type 1 Diabetes.Front Endocrinol (Lausanne). 2022 Apr 13;13:883000. doi: 10.3389/fendo.2022.883000. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35498419 Free PMC article. Review.
-
Immunopeptidome mining reveals a novel ERS-induced target in T1D.Cell Mol Immunol. 2024 Jun;21(6):604-619. doi: 10.1038/s41423-024-01150-0. Epub 2024 Apr 30. Cell Mol Immunol. 2024. PMID: 38689020 Free PMC article.
-
A multi-epitope DNA vaccine enables a broad engagement of diabetogenic T cells for tolerance in Type 1 diabetes.J Autoimmun. 2019 Mar;98:13-23. doi: 10.1016/j.jaut.2018.11.003. Epub 2018 Nov 17. J Autoimmun. 2019. PMID: 30454875 Free PMC article.
-
The role of islet autoantigen-specific T cells in the onset and treatment of type 1 diabetes mellitus.Front Immunol. 2024 Sep 24;15:1462384. doi: 10.3389/fimmu.2024.1462384. eCollection 2024. Front Immunol. 2024. PMID: 39380988 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

