BAG3 Protein Is Involved in Endothelial Cell Response to Phenethyl Isothiocyanate
- PMID: 29955247
- PMCID: PMC6000881
- DOI: 10.1155/2018/5967890
BAG3 Protein Is Involved in Endothelial Cell Response to Phenethyl Isothiocyanate
Abstract
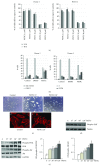
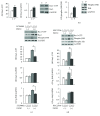
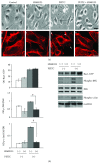
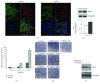
Phenethyl isothiocyanate (PEITC), a cruciferous vegetable-derived compound, is a versatile cancer chemopreventive agent that displays the ability to inhibit tumor growth during initiation, promotion, and progression phases in several animal models of carcinogenesis. In this report, we dissect the cellular events induced by noncytotoxic concentrations of PEITC in human umbilical vein endothelial cells (HUVECs). In the early phase, PEITC treatment elicited cells' morphological changes that comprise reduction in cell volume and modification of actin organization concomitantly with a rapid activation of the PI3K/Akt pathway. Downstream to PI3K, PEITC also induces the activity of Rac1 and activation of c-Jun N-terminal kinase (JNK), well-known regulators of actin cytoskeleton dynamics. Interestingly, PEITC modifications of the actin cytoskeleton were abrogated by pretreatment with JNK inhibitor, SP600125. JNK signaling led also to the activation of the c-Jun transcription factor, which is involved in the upregulation of several genes; among them is the BAG3 protein. This protein, a member of the BAG family of heat shock protein (Hsp) 70 cochaperones, is able to sustain survival in different tumor cell lines and neoangiogenesis by directly regulating the endothelial cell cycle. Furthermore, BAG3 is involved in maintaining actin folding. Our findings indicate that BAG3 protein expression is induced in endothelial cells upon exposure to a noncytotoxic concentration of PEITC and its expression is requested for the recovery of normal cell size and morphology after the stressful stimuli. This assigns an additional role for BAG3 protein in the endothelial cells after a stress event.
Figures
Similar articles
-
c-jun/AP-1 activation does not affect the antiproliferative activity of phenethyl isothiocyanate, a cruciferous vegetable-derived cancer chemopreventive agent.Mol Carcinog. 2006 Aug;45(8):605-12. doi: 10.1002/mc.20206. Mol Carcinog. 2006. PMID: 16652374
-
Phenethyl isothiocyanate, a natural chemopreventive agent, activates c-Jun N-terminal kinase 1.Cancer Res. 1996 Jul 1;56(13):2954-9. Cancer Res. 1996. PMID: 8674048
-
Phenethyl isothiocyanate, a cancer chemopreventive constituent of cruciferous vegetables, inhibits cap-dependent translation by regulating the level and phosphorylation of 4E-BP1.Cancer Res. 2007 Apr 15;67(8):3569-73. doi: 10.1158/0008-5472.CAN-07-0392. Cancer Res. 2007. PMID: 17440067
-
Advances in molecular signaling mechanisms of β-phenethyl isothiocyanate antitumor effects.J Agric Food Chem. 2015 Apr 8;63(13):3311-22. doi: 10.1021/jf504627e. Epub 2015 Mar 30. J Agric Food Chem. 2015. PMID: 25798652 Review.
-
Role of BAG3 in cancer progression: A therapeutic opportunity.Semin Cell Dev Biol. 2018 Jun;78:85-92. doi: 10.1016/j.semcdb.2017.08.049. Epub 2017 Aug 31. Semin Cell Dev Biol. 2018. PMID: 28864347 Review.
Cited by
-
BAG3 expression and sarcomere localization in the human heart are linked to HSF-1 and are differentially affected by sex and disease.Am J Physiol Heart Circ Physiol. 2021 Jun 1;320(6):H2339-H2350. doi: 10.1152/ajpheart.00419.2020. Epub 2021 May 14. Am J Physiol Heart Circ Physiol. 2021. PMID: 33989081 Free PMC article.
-
Lumacaftor and Matrine: Possible Therapeutic Combination to Counteract the Inflammatory Process in Cystic Fibrosis.Biomolecules. 2021 Mar 13;11(3):422. doi: 10.3390/biom11030422. Biomolecules. 2021. PMID: 33805605 Free PMC article.
-
Structural Refinement of 2,4-Thiazolidinedione Derivatives as New Anticancer Agents Able to Modulate the BAG3 Protein.Molecules. 2022 Jan 20;27(3):665. doi: 10.3390/molecules27030665. Molecules. 2022. PMID: 35163936 Free PMC article.
-
Alarmins and c-Jun N-Terminal Kinase (JNK) Signaling in Neuroinflammation.Cells. 2020 Oct 24;9(11):2350. doi: 10.3390/cells9112350. Cells. 2020. PMID: 33114371 Free PMC article. Review.
-
Nanoengineered hydrogels disrupt tumor antioxidant defense via photothermal-chemodynamic synergy and oxidative stress boosts.J Nanobiotechnology. 2025 Aug 23;23(1):584. doi: 10.1186/s12951-025-03626-1. J Nanobiotechnology. 2025. PMID: 40849478 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

