Assessment of the Validity of Nuclear-Localized Androgen Receptor Splice Variant 7 in Circulating Tumor Cells as a Predictive Biomarker for Castration-Resistant Prostate Cancer
- PMID: 29955787
- PMCID: PMC6139066
- DOI: 10.1001/jamaoncol.2018.1621
Assessment of the Validity of Nuclear-Localized Androgen Receptor Splice Variant 7 in Circulating Tumor Cells as a Predictive Biomarker for Castration-Resistant Prostate Cancer
Abstract
Importance: A blood test to determine whether to treat patients with metastatic castration-resistant prostate cancer (mCRPC) with an androgen receptor signaling (ARS) inhibitor or taxane is an unmet medical need.
Objective: To determine whether a validated assay for the nuclear-localized androgen receptor splice variant 7 (AR-V7) protein in circulating tumor cells can determine differential overall survival among patients with mCRPC treated with taxanes vs ARS inhibitors.
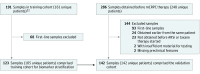
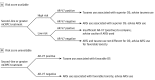
Design, setting, and participants: This blinded correlative study conducted from December 31, 2012, to September 1, 2016, included 142 patients with histologically confirmed mCRPC and who were treated at Memorial Sloan Kettering Cancer Center, The Royal Marsden, or the London Health Sciences Centre. Blood samples were obtained prior to administration of ARS inhibitors or taxanes as a second-line or greater systemic therapy for progressing mCRPC.
Main outcomes and measures: Overall survival after treatment with an ARS inhibitor or taxane in relation to pretherapy AR-V7 status.
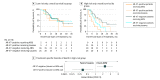
Results: Among the 142 patients in the study (mean [SD] age, 69.5 [9.6] years), 70 were designated as high risk by conventional prognostic factors. In this high-risk group, patients positive for AR-V7 who were treated with taxanes had superior overall survival relative to those treated with ARS inhibitors (median overall survival, 14.3 vs 7.3 months; hazard ratio, 0.62; 95% CI, 0.28-1.39; P = .25). Patients negative for AR-V7 who were treated with ARS inhibitors had superior overall survival relative to those treated with taxanes (median overall survival, 19.8 vs 12.8 months; hazard ratio, 1.67; 95% CI, 1.00-2.81; P = .05).
Conclusions and relevance: This study suggests that nuclear-localized AR-V7 protein in circulating tumor cells can identify patients who may live longer with taxane chemotherapy vs ARS inhibitor treatment.
Conflict of interest statement
Figures

Comment in
-
Nuclear Circulating Tumor Cell Androgen Receptor Variant 7 in Castration-Resistant Prostate Cancer: The Devil Is in the Detail.JAMA Oncol. 2018 Sep 1;4(9):1187-1188. doi: 10.1001/jamaoncol.2018.1615. JAMA Oncol. 2018. PMID: 29955776 No abstract available.
-
AR-V7 detection guides treatment.Nat Rev Clin Oncol. 2018 Sep;15(9):533. doi: 10.1038/s41571-018-0072-5. Nat Rev Clin Oncol. 2018. PMID: 30018408 No abstract available.
-
AR-V7 and treatment selection in advanced prostate cancer: are we there yet?Precis Cancer Med. 2018 Sep;1:13. doi: 10.21037/pcm.2018.09.01. Epub 2018 Sep 18. Precis Cancer Med. 2018. PMID: 30370427 Free PMC article. No abstract available.
-
Improving the Nuclear-Localized Androgen Receptor Splice Variant 7 Test-In Reply.JAMA Oncol. 2019 Mar 1;5(3):434. doi: 10.1001/jamaoncol.2018.6686. JAMA Oncol. 2019. PMID: 30703186 No abstract available.
-
Improving the Nuclear-Localized Androgen Receptor Splice Variant 7 Test.JAMA Oncol. 2019 Mar 1;5(3):433-434. doi: 10.1001/jamaoncol.2018.6683. JAMA Oncol. 2019. PMID: 30703189 No abstract available.
References
-
- Gillessen S, Attard G, Beer TM, et al. Management of patients with advanced prostate cancer: the report of the Advanced Prostate Cancer Consensus Conference APCCC 2017. Eur Urol. 2018;73(2):178-211. - PubMed
-
- Schrader AJ, Boegemann M, Ohlmann CH, et al. Enzalutamide in castration-resistant prostate cancer patients progressing after docetaxel and abiraterone. Eur Urol. 2014;65(1):30-36. - PubMed
-
- de Bono JS, Chowdhury S, Feyerabend S, et al. Antitumour activity and safety of enzalutamide in patients with metastatic castration-resistant prostate cancer previously treated with abiraterone acetate plus prednisone for ≥24 weeks in Europe [published online August 22, 2017]. Eur Urol. doi: 10.1016/j.eururo.2017.07.035 - DOI - PubMed
-
- Aggarwal RR, Feng FY, Small EJ. Emerging categories of disease in advanced prostate cancer and their therapeutic implications. Oncology (Williston Park). 2017;31(6):467-474. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

