MBNL splicing activity depends on RNA binding site structural context
- PMID: 29955876
- PMCID: PMC6158504
- DOI: 10.1093/nar/gky565
MBNL splicing activity depends on RNA binding site structural context
Abstract
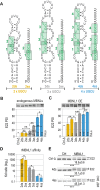
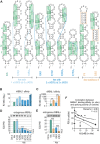
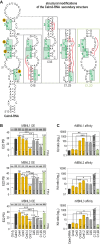
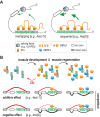
Muscleblind-like (MBNL) proteins are conserved RNA-binding factors involved in alternative splicing (AS) regulation during development. While AS is controlled by distribution of MBNL paralogs and isoforms, the affinity of these proteins for specific RNA-binding regions and their location within transcripts, it is currently unclear how RNA structure impacts MBNL-mediated AS regulation. Here, we defined the RNA structural determinants affecting MBNL-dependent AS activity using both cellular and biochemical assays. While enhanced inclusion of MBNL-regulated alternative exons is controlled by the arrangement and number of MBNL binding sites within unstructured RNA, when these sites are embedded in a RNA hairpin MBNL binds preferentially to one side of stem region. Surprisingly, binding of MBNL proteins to RNA targets did not entirely correlate with AS efficiency. Moreover, comparison of MBNL proteins revealed structure-dependent competitive behavior between the paralogs. Our results showed that the structure of targeted RNAs is a prevalent component of the mechanism of alternative splicing regulation by MBNLs.
Figures



References
-
- Kornblihtt A.R., Schor I.E., Allo M., Dujardin G., Petrillo E., Munoz M.J.. Alternative splicing: a pivotal step between eukaryotic transcription and translation. Nat. Rev. Mol. Cell Biol. 2013; 14:153–165. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

