Characterization of a new semi-dominant dwarf allele of SLR1 and its potential application in hybrid rice breeding
- PMID: 29955878
- PMCID: PMC6137977
- DOI: 10.1093/jxb/ery243
Characterization of a new semi-dominant dwarf allele of SLR1 and its potential application in hybrid rice breeding
Abstract
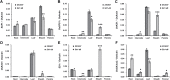
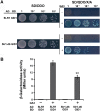
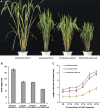
The widespread introduction of semi-dwarf1 (sd1), also known as the 'Green Revolution' gene, has dramatically increased rice yield. However, the extensive use of limited sources of dwarf genes may cause 'bottleneck' effects in breeding new rice varieties. Alternative dwarf germplasms are quite urgent for rice breeding. Here, we characterized a new allele of the rice Slr1-d mutant, Slr1-d6, which reduced plant height by 37%, a much milder allele for dwarfism. Slr-d6 was still responsive to gibberellin (GA) to a reduced extent. The mutation site in Slr1-d6 was less conserved in the TVHYNP domain, leading to the specific semi-dominant dwarf phenotype. Expression of SLR1 and five key GA biosynthetic genes was disturbed in Slr1-d6, and the interaction between Slr1-d6 and GID1 was decreased. In the genetic background of cultivar 9311 with sd1 eliminated, Slr1-d6 homozygous plants were ~70 cm tall. Moreover, Slr1-d6 heterozygous plants were equivalent in height to the standard sd1 semi-dwarf 9311, but with a 25% yield increase, showing its potential application in hybrid rice breeding.
Figures


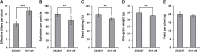
References
-
- Asano K, Hirano K, Ueguchi-Tanaka M, Angeles-Shim RB, Komura T, Satoh H, Kitano H, Matsuoka M, Ashikari M. 2009. Isolation and characterization of dominant dwarf mutants, Slr1-d, in rice. Molecular Genetics and Genomics 281, 223–231. - PubMed
-
- Asano K, Takashi T, Miura K, Qian Q, Kitano H, Matsuoka M, Ashikari M. 2007. Genetic and molecular analysis of utility of sd1 alleles in rice breeding. Breeding Science 57, 53–58.
-
- Chomczynski P, Mackey K. 1995. Short technical reports. Modification of the TRI reagent procedure for isolation of RNA from polysaccharide- and proteoglycan-rich sources. Biotechniques 19, 942–945. - PubMed
-
- Davière JM, Achard P. 2013. Gibberellin signaling in plants. Development 140, 1147–1151. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

