Toxicity, recovery, and resilience in a 3D dopaminergic neuronal in vitro model exposed to rotenone
- PMID: 29955902
- PMCID: PMC6063347
- DOI: 10.1007/s00204-018-2250-8
Toxicity, recovery, and resilience in a 3D dopaminergic neuronal in vitro model exposed to rotenone
Abstract
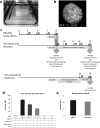
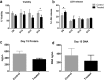
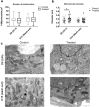
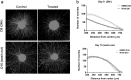
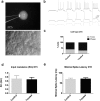
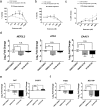
To date, most in vitro toxicity testing has focused on acute effects of compounds at high concentrations. This testing strategy does not reflect real-life exposures, which might contribute to long-term disease outcome. We used a 3D-human dopaminergic in vitro LUHMES cell line model to determine whether effects of short-term rotenone exposure (100 nM, 24 h) are permanent or reversible. A decrease in complex I activity, ATP, mitochondrial diameter, and neurite outgrowth were observed acutely. After compound removal, complex I activity was still inhibited; however, ATP levels were increased, cells were electrically active and aggregates restored neurite outgrowth integrity and mitochondrial morphology. We identified significant transcriptomic changes after 24 h which were not present 7 days after wash-out. Our results suggest that testing short-term exposures in vitro may capture many acute effects which cells can overcome, missing adaptive processes, and long-term mechanisms. In addition, to study cellular resilience, cells were re-exposed to rotenone after wash-out and recovery period. Pre-exposed cells maintained higher metabolic activity than controls and presented a different expression pattern in genes previously shown to be altered by rotenone. NEF2L2, ATF4, and EAAC1 were downregulated upon single hit on day 14, but unchanged in pre-exposed aggregates. DAT and CASP3 were only altered after re-exposure to rotenone, while TYMS and MLF1IP were downregulated in both single-exposed and pre-exposed aggregates. In summary, our study shows that a human cell-based 3D model can be used to assess cellular adaptation, resilience, and long-term mechanisms relevant to neurodegenerative research.
Keywords: 3D LUHMES; Cellular defence; Gene response; Neurodegeneration; Pesticide; Recovery; Resilience; Rotenone.
Conflict of interest statement
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This article does not contain any clinical studies with human participants or animals performed by any of the authors.
Figures
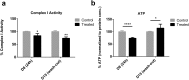

References
-
- Bal-Price A, Crofton KM, Sachana M, Shafer TJ, Behl M, Forsby A, Hargreaves A, Landesmann B, Lein PJ, Louisse J, Monnet-Tschudi F, Paini A, Rolaki A, Schrattenholz A, Suñol C, van Thriel C, Whelan M, Fritsche E. Putative adverse outcome pathways relevant to neurotoxicity. Crit Rev Toxicol. 2015;45(1):83–91. doi: 10.3109/10408444.2014.981331. - DOI - PMC - PubMed
-
- Bal-Price A, Leist M, Schildknecht S, Tschudi-Monnet F, Paini A, Terron A (2017b) Inhibition of the mitochondrial complex I of nigro-striatal neurons leads to parkinsonian motor deficits. https://aopwiki.org/aops/3. Accessed 25 Feb 2018
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

