Spatio-temporal release of NGF and GDNF from multi-layered nanofibrous bicomponent electrospun scaffolds
- PMID: 29955977
- PMCID: PMC6022522
- DOI: 10.1007/s10856-018-6105-x
Spatio-temporal release of NGF and GDNF from multi-layered nanofibrous bicomponent electrospun scaffolds
Abstract
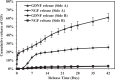
Scaffolds capable of providing dual neurotrophic factor (NTF) delivery with different release kinetics, spatial delivery of NTFs at different loci and topographical guidance are promising for enhanced peripheral nerve regeneration. In this study, we have designed and fabricated multi-layered aligned-fiber scaffolds through combining emulsion electrospinning, sequential electrospinning and high-speed electrospinning (HS-ES) to modulate the release behavior of glial cell line-derived growth factor(GDNF) and nerve growth factor (NGF). GDNF and NGF were incorporated into poly(lactic-co-glycolic acid) (PLGA) fibers and poly(D,L-lactic acid) (PDLLA) fibers, respectively. Aligned fibers were obtained in each layer of multi-layered scaffolds and relatively thick tri-layered and tetra-layered scaffolds with controlled layer thickness were obtained. Their morphology, structure, properties, and the in vitro release of growth factors were examined. Dual and spatio-temporal release of GDNF and NGF with different release kinetics from multi-layered scaffolds was successfully demonstrated. High separation efficiency by PDLLA fibrous barrier layer for spatial neurotrophic factor delivery from both tri-layered scaffolds and tetra-layered scaffolds was achieved.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures











Similar articles
-
Nanofibrous bicomponent scaffolds for the dual delivery of NGF and GDNF: controlled release of growth factors and their biological effects.J Mater Sci Mater Med. 2021 Jan 20;32(1):9. doi: 10.1007/s10856-020-06479-2. J Mater Sci Mater Med. 2021. PMID: 33471206 Free PMC article.
-
Incorporation and release of dual growth factors for nerve tissue engineering using nanofibrous bicomponent scaffolds.Biomed Mater. 2018 May 4;13(4):044107. doi: 10.1088/1748-605X/aab693. Biomed Mater. 2018. PMID: 29537390
-
Heparin/collagen encapsulating nerve growth factor multilayers coated aligned PLLA nanofibrous scaffolds for nerve tissue engineering.J Biomed Mater Res A. 2017 Jul;105(7):1900-1910. doi: 10.1002/jbm.a.36053. Epub 2017 Apr 3. J Biomed Mater Res A. 2017. PMID: 28256802
-
Nerve conduit scaffolds for discrete delivery of two neurotrophic factors.Eur J Pharm Biopharm. 2013 Sep;85(1):139-42. doi: 10.1016/j.ejpb.2013.03.030. Eur J Pharm Biopharm. 2013. PMID: 23958324
-
Dual-delivery of VEGF and NGF by emulsion electrospun nanofibrous scaffold for peripheral nerve regeneration.Mater Sci Eng C Mater Biol Appl. 2018 Jan 1;82:253-264. doi: 10.1016/j.msec.2017.08.030. Epub 2017 Aug 12. Mater Sci Eng C Mater Biol Appl. 2018. PMID: 29025656
Cited by
-
Multi-material electrospinning: from methods to biomedical applications.Mater Today Bio. 2023 Jun 23;21:100710. doi: 10.1016/j.mtbio.2023.100710. eCollection 2023 Aug. Mater Today Bio. 2023. PMID: 37545561 Free PMC article. Review.
-
Electrospun Fiber Scaffolds for Engineering Glial Cell Behavior to Promote Neural Regeneration.Bioengineering (Basel). 2020 Dec 29;8(1):4. doi: 10.3390/bioengineering8010004. Bioengineering (Basel). 2020. PMID: 33383759 Free PMC article. Review.
-
Nanofibrous bicomponent scaffolds for the dual delivery of NGF and GDNF: controlled release of growth factors and their biological effects.J Mater Sci Mater Med. 2021 Jan 20;32(1):9. doi: 10.1007/s10856-020-06479-2. J Mater Sci Mater Med. 2021. PMID: 33471206 Free PMC article.
-
An emulsion electrospun nanofibrous scaffold loaded with glial cell line-derived neurotrophic factor for nerve regeneration.Front Cell Dev Biol. 2025 Apr 16;13:1567654. doi: 10.3389/fcell.2025.1567654. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40309241 Free PMC article.
-
Sustained Biochemical Signaling and Contact Guidance by Electrospun Bicomponents as Promising Scaffolds for Nerve Tissue Regeneration.ACS Omega. 2021 Nov 24;6(48):33010-33017. doi: 10.1021/acsomega.1c05117. eCollection 2021 Dec 7. ACS Omega. 2021. PMID: 34901652 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

