Tuning the endothelial response: differential release of exocytic cargos from Weibel-Palade bodies
- PMID: 29956444
- PMCID: PMC6166140
- DOI: 10.1111/jth.14218
Tuning the endothelial response: differential release of exocytic cargos from Weibel-Palade bodies
Abstract
Essentials Endothelial activation initiates multiple processes, including hemostasis and inflammation. The molecules that contribute to these processes are co-stored in secretory granules. How can the cells control release of granule content to allow differentiated responses? Selected agonists recruit an exocytosis-linked actin ring to boost release of a subset of cargo.
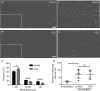
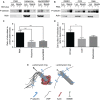
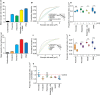
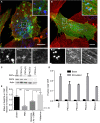
Summary: Background Endothelial cells harbor specialized storage organelles, Weibel-Palade bodies (WPBs). Exocytosis of WPB content into the vascular lumen initiates primary hemostasis, mediated by von Willebrand factor (VWF), and inflammation, mediated by several proteins including P-selectin. During full fusion, secretion of this large hemostatic protein and smaller pro-inflammatory proteins are thought to be inextricably linked. Objective To determine if secretagogue-dependent differential release of WPB cargo occurs, and whether this is mediated by the formation of an actomyosin ring during exocytosis. Methods We used VWF string analysis, leukocyte rolling assays, ELISA, spinning disk confocal microscopy, high-throughput confocal microscopy and inhibitor and siRNA treatments to demonstrate the existence of cellular machinery that allows differential release of WPB cargo proteins. Results Inhibition of the actomyosin ring differentially effects two processes regulated by WPB exocytosis; it perturbs VWF string formation but has no effect on leukocyte rolling. The efficiency of ring recruitment correlates with VWF release; the ratio of release of VWF to small cargoes decreases when ring recruitment is inhibited. The recruitment of the actin ring is time dependent (fusion events occurring directly after stimulation are less likely to initiate hemostasis than later events) and is activated by protein kinase C (PKC) isoforms. Conclusions Secretagogues differentially recruit the actomyosin ring, thus demonstrating one mechanism by which the prothrombotic effect of endothelial activation can be modulated. This potentially limits thrombosis whilst permitting a normal inflammatory response. These results have implications for the assessment of WPB fusion, cargo-content release and the treatment of patients with von Willebrand disease.
Keywords: Weibel-Palade bodies; exocytosis; hemostasis; inflammation; von Willebrand factor.
© 2018 The Authors. Journal of Thrombosis and Haemostasis published by Wiley Periodicals, Inc. on behalf of International Society on Thrombosis and Haemostasis.
Figures



References
-
- Metcalf DJ, Nightingale TD, Zenner HL, Lui‐Roberts WW, Cutler DF. Formation and function of Weibel‐Palade bodies. J Cell Sci 2008; 121: 19–27. - PubMed
-
- Rondaij MG, Bierings R, Kragt A, van Mourik JA, Voorberg J. Dynamics and plasticity of Weibel‐Palade bodies in endothelial cells. Arterioscler Thromb Vasc Biol 2006; 26: 1002–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

