Effects of liraglutide, metformin and gliclazide on body composition in patients with both type 2 diabetes and non-alcoholic fatty liver disease: A randomized trial
- PMID: 29957886
- PMCID: PMC6400178
- DOI: 10.1111/jdi.12888
Effects of liraglutide, metformin and gliclazide on body composition in patients with both type 2 diabetes and non-alcoholic fatty liver disease: A randomized trial
Abstract
Aims/introduction: To compare the effects of gliclazide, liraglutide and metformin on body composition in patients with type 2 diabetes mellitus with non-alcoholic fatty liver disease.
Materials and methods: A total of 85 patients were randomly allocated to receive gliclazide (n = 27), liraglutide (n = 29) or metformin (n = 29) monotherapy for 24 weeks. Body composition was measured using dual-energy X-ray absorptiometry.
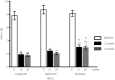
Results: Liraglutide and metformin reduced total, trunk, limb, android and gynoid fat mass; this also led to weight reduction. However, gliclazide treatment produced no significant changes in weight or fat mass, likely because reductions in fat mass were concomitant with increases in lean tissue mass. Blood glucose concentrations and glycated hemoglobin levels improved in all treatment arms; levels of the latter were lower in patients treated with liraglutide and metformin. Serum alanine aminotransferase concentrations decreased in all treatment arms, whereas serum aspartate aminotransferase concentrations were reduced only by liraglutide and metformin. In all patients, weight loss and total, trunk, limb, and android fat mass reductions were positively correlated with decreases in serum alanine aminotransferase and aspartate aminotransferase levels, whereas reductions in waist circumference were positively correlated with lower serum alanine aminotransferase levels.
Conclusions: Compared with gliclazide, liraglutide and metformin monotherapies result in greater weight loss, reductions in body fat mass, and better blood glucose control among type 2 diabetes mellitus patients with non-alcoholic fatty liver disease. Reductions in weight, fat mass and waist circumference favorably affect hepatic function.
Keywords: Antidiabetic agents; Body composition; Type 2 diabetes mellitus.
© 2018 The Authors. Journal of Diabetes Investigation published by Asian Association for the Study of Diabetes (AASD) and John Wiley & Sons Australia, Ltd.
Figures


References
-
- European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO) . EASL‐EASD‐EASO Clinical Practice Guidelines for the management of non‐alcoholic fatty liver disease. J Hepatol 2016; 64: 1388–1402. - PubMed
-
- American Diabetes Association . Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes‐2018. Diabetes Care 2018; 41(Suppl 1): S65–S72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 81570737/National Natural Science Foundation of China
- 81570736/National Natural Science Foundation of China
- 81370947/National Natural Science Foundation of China
- Project of National Key Clinical Division
- XK201105/Jiangsu Province's Key Discipline of Medicine
- YKK14055/Medical and Health Research Projects of Nanjing Health Bureau in Jiangsu Province of China
- JQX13010/Nanjing Outstanding Youth Fund Projects in Jiangsu Province of China
- 2013ZD005/Nanjing Science and Technology Development projects in Jiangsu province of China
- 2015604/Project of Standardized Diagnosis and Treatment of Key Diseases in Jiangsu province of China
- 2017-N-05/China Diabetes Young Scientific Talent Research Project
- 14380296/Nanjing University Central University Basic Scientific Research
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

