Computation predicts rapidly adapting mechanotransduction currents cannot account for tactile encoding in Merkel cell-neurite complexes
- PMID: 29958280
- PMCID: PMC6042796
- DOI: 10.1371/journal.pcbi.1006264
Computation predicts rapidly adapting mechanotransduction currents cannot account for tactile encoding in Merkel cell-neurite complexes
Abstract
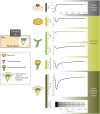
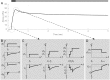
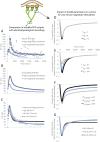
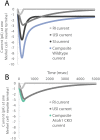
Distinct firing properties among touch receptors are influenced by multiple, interworking anatomical structures. Our understanding of the functions and crosstalk of Merkel cells and their associated neurites-the end organs of slowly adapting type I (SAI) afferents-remains incomplete. Piezo2 mechanically activated channels are required both in Merkel cells and in sensory neurons for canonical SAI responses in rodents; however, a central unanswered question is how rapidly inactivating currents give rise to sustained action potential volleys in SAI afferents. The computational model herein synthesizes mechanotransduction currents originating from Merkel cells and neurites, in context of skin mechanics and neural dynamics. Its goal is to mimic distinct spike firing patterns from wildtype animals, as well as Atoh1 knockout animals that completely lack Merkel cells. The developed generator function includes a Merkel cell mechanism that represents its mechanotransduction currents and downstream voltage-activated conductances (slower decay of current) and a neurite mechanism that represents its mechanotransduction currents (faster decay of current). To mimic sustained firing in wildtype animals, a longer time constant was needed than the 200 ms observed for mechanically activated membrane depolarizations in rodent Merkel cells. One mechanism that suffices is to introduce an ultra-slowly inactivating current, with a time constant on the order of 1.7 s. This mechanism may drive the slow adaptation of the sustained response, for which the skin's viscoelastic relaxation cannot account. Positioned within the sensory neuron, this source of current reconciles the physiology and anatomical characteristics of Atoh1 knockout animals.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Johnson K. The roles and functions of cutaneous mechanoreceptors. Current Opinion in Neurobiology. 2001. August 1;11(4):455–61. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

