Innate immune sensor LGP2 is cleaved by the Leader protease of foot-and-mouth disease virus
- PMID: 29958302
- PMCID: PMC6042790
- DOI: 10.1371/journal.ppat.1007135
Innate immune sensor LGP2 is cleaved by the Leader protease of foot-and-mouth disease virus
Abstract
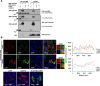
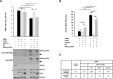
The RNA helicase LGP2 (Laboratory of Genetics and Physiology 2) is a non-signaling member of the retinoic acid-inducible gene-I (RIG-I)-like receptors (RLRs), whose pivotal role on innate immune responses against RNA viruses is being increasingly uncovered. LGP2 is known to work in synergy with melanoma differentiation-associated gene 5 (MDA5) to promote the antiviral response induced by picornavirus infection. Here, we describe the activity of the foot-and-mouth disease virus (FMDV) Leader protease (Lpro) targeting LGP2 for cleavage. When LGP2 and Lpro were co-expressed, cleavage products were observed in an Lpro dose-dependent manner while co-expression with a catalytically inactive Lpro mutant had no effect on LGP2 levels or pattern. We further show that Lpro localizes and immunoprecipitates with LGP2 in transfected cells supporting their interaction within the cytoplasm. Evidence of LGP2 proteolysis was also detected during FMDV infection. Moreover, the inhibitory effect of LGP2 overexpression on FMDV growth observed was reverted when Lpro was co-expressed, concomitant with lower levels of IFN-β mRNA and antiviral activity in those cells. The Lpro target site in LGP2 was identified as an RGRAR sequence in a conserved helicase motif whose replacement to EGEAE abrogated LGP2 cleavage by Lpro. Taken together, these data suggest that LGP2 cleavage by the Leader protease of aphthoviruses may represent a novel antagonistic mechanism for immune evasion.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Uncovering targets of the Leader protease: Linking RNA-mediated pathways and antiviral defense.Wiley Interdiscip Rev RNA. 2021 Jul;12(4):e1645. doi: 10.1002/wrna.1645. Epub 2021 Feb 18. Wiley Interdiscip Rev RNA. 2021. PMID: 33605051 Free PMC article. Review.
-
MDA5 cleavage by the Leader protease of foot-and-mouth disease virus reveals its pleiotropic effect against the host antiviral response.Cell Death Dis. 2020 Sep 2;11(8):718. doi: 10.1038/s41419-020-02931-x. Cell Death Dis. 2020. PMID: 32879301 Free PMC article.
-
Impairment of the DeISGylation Activity of Foot-and-Mouth Disease Virus Lpro Causes Attenuation In Vitro and In Vivo.J Virol. 2020 Jun 16;94(13):e00341-20. doi: 10.1128/JVI.00341-20. Print 2020 Jun 16. J Virol. 2020. PMID: 32295921 Free PMC article.
-
Inhibition of Antiviral Innate Immunity by Foot-and-Mouth Disease Virus Lpro through Interaction with the N-Terminal Domain of Swine RNase L.J Virol. 2021 Jul 12;95(15):e0036121. doi: 10.1128/JVI.00361-21. Epub 2021 Jul 12. J Virol. 2021. PMID: 33980594 Free PMC article.
-
Molecular Mechanisms of Foot-and-Mouth Disease Virus Targeting the Host Antiviral Response.Front Cell Infect Microbiol. 2017 Jun 13;7:252. doi: 10.3389/fcimb.2017.00252. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28660175 Free PMC article. Review.
Cited by
-
The DEAD-Box RNA Helicase DDX1 Interacts with the Viral Protein 3D and Inhibits Foot-and-Mouth Disease Virus Replication.Virol Sin. 2019 Dec;34(6):610-617. doi: 10.1007/s12250-019-00148-7. Epub 2019 Jul 29. Virol Sin. 2019. PMID: 31359346 Free PMC article.
-
Uncovering targets of the Leader protease: Linking RNA-mediated pathways and antiviral defense.Wiley Interdiscip Rev RNA. 2021 Jul;12(4):e1645. doi: 10.1002/wrna.1645. Epub 2021 Feb 18. Wiley Interdiscip Rev RNA. 2021. PMID: 33605051 Free PMC article. Review.
-
Porcine Deltacoronavirus nsp5 Cleaves DCP1A To Decrease Its Antiviral Activity.J Virol. 2020 Jul 16;94(15):e02162-19. doi: 10.1128/JVI.02162-19. Print 2020 Jul 16. J Virol. 2020. PMID: 32461317 Free PMC article.
-
Foot-and-Mouth Disease Virus 3B Protein Interacts with Pattern Recognition Receptor RIG-I to Block RIG-I-Mediated Immune Signaling and Inhibit Host Antiviral Response.J Immunol. 2020 Oct 15;205(8):2207-2221. doi: 10.4049/jimmunol.1901333. Epub 2020 Sep 11. J Immunol. 2020. PMID: 32917788 Free PMC article.
-
Molecular Mechanisms of Immune Escape for Foot-and-Mouth Disease Virus.Pathogens. 2020 Sep 4;9(9):729. doi: 10.3390/pathogens9090729. Pathogens. 2020. PMID: 32899635 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

