Refinement and Analysis of the Mature Zika Virus Cryo-EM Structure at 3.1 Å Resolution
- PMID: 29958768
- PMCID: PMC6125166
- DOI: 10.1016/j.str.2018.05.006
Refinement and Analysis of the Mature Zika Virus Cryo-EM Structure at 3.1 Å Resolution
Abstract
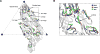
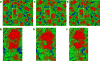
Among the several arthropod-borne human flaviviral diseases, the recent outbreak of Zika virus (ZIKV) has caused devastating birth defects and neurological disorders, challenging the world with another major public health concern. We report here the refined structure of the mature ZIKV at a resolution of 3.1 Å as determined by cryo-electron microscopic single-particle reconstruction. The improvement in the resolution, compared with previous enveloped virus structures, was the result of optimized virus preparation methods and data processing techniques. The glycoprotein interactions and surface properties of ZIKV were compared with other mosquito-borne flavivirus structures. The largest structural differences and sequence variations occur at the glycosylation loop associated with receptor binding. Probable drug binding pockets were identified on the viral surface. These results also provide a structural basis for the design of vaccines against ZIKV.
Keywords: Zika virus; antiviral binding sites; flavivirus comparisons; receptor binding sites; structure refinement.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Barba-Spaeth G, Dejnirattisai W, Rouvinski A, Vaney MC, Medits I, Sharma A, Simon-Loriere E, Sakuntabhai A, Cao-Lormeau VM, Haouz A, et al. Structural basis of potent Zika-dengue virus antibody cross-neutralization. Nature. 2016;536:48–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

