Sympathetic Neuronal Activation Triggers Myeloid Progenitor Proliferation and Differentiation
- PMID: 29958804
- PMCID: PMC6051926
- DOI: 10.1016/j.immuni.2018.05.004
Sympathetic Neuronal Activation Triggers Myeloid Progenitor Proliferation and Differentiation
Abstract
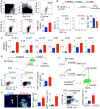
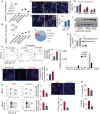
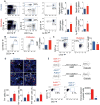
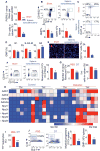
There is a growing body of research on the neural control of immunity and inflammation. However, it is not known whether the nervous system can regulate the production of inflammatory myeloid cells from hematopoietic progenitor cells in disease conditions. Myeloid cell numbers in diabetic patients were strongly correlated with plasma concentrations of norepinephrine, suggesting the role of sympathetic neuronal activation in myeloid cell production. The spleens of diabetic patients and mice contained higher numbers of tyrosine hydroxylase (TH)-expressing leukocytes that produced catecholamines. Granulocyte macrophage progenitors (GMPs) expressed the β2 adrenergic receptor, a target of catecholamines. Ablation of splenic sympathetic neuronal signaling using surgical, chemical, and genetic approaches diminished GMP proliferation and myeloid cell development. Finally, mice lacking TH-producing leukocytes had reduced GMP proliferation, resulting in diminished myelopoiesis. Taken together, our study demonstrates that catecholamines produced by leukocytes and sympathetic nerve termini promote GMP proliferation and myeloid cell development.
Keywords: GMP; adrenergic receptors; atherosclerosis; catecholamines; diabetes; myeloid progenitors; myelopoiesis; neuropeptide Y receptor; norepinephrine; sympathetic neuronal activation.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest: The authors have declared that no conflict of interest exists.
Figures



Comment in
-
Splenic Leukocytes Get the Nerves up for Myelopoiesis.Immunity. 2018 Jul 17;49(1):1-3. doi: 10.1016/j.immuni.2018.06.014. Immunity. 2018. PMID: 30021139
References
-
- Bokhari S, Plummer E, Emmerson P, Gupta A, Meyer C. Glucose counterregulation in advanced type 2 diabetes: effect of beta-adrenergic blockade. Diabetes Care. 2014;37:3040–3046. - PubMed
-
- Brown SW, Meyers RT, Brennan KM, Rumble JM, Narasimhachari N, Perozzi EF, Ryan JJ, Stewart JK, Fischer-Stenger K. Catecholamines in a macrophage cell line. J Neuroimmunol. 2003;135:47–55. - PubMed
-
- Carnevale D, Pallante F, Fardella V, Fardella S, Iacobucci R, Federici M, Cifelli G, De Lucia M, Lembo G. The angiogenic factor PlGF mediates a neuroimmune interaction in the spleen to allow the onset of hypertension. Immunity. 2014;41:737–752. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

