Glycyrrhizin Alleviates Nonalcoholic Steatohepatitis via Modulating Bile Acids and Meta-Inflammation
- PMID: 29959134
- PMCID: PMC6081736
- DOI: 10.1124/dmd.118.082008
Glycyrrhizin Alleviates Nonalcoholic Steatohepatitis via Modulating Bile Acids and Meta-Inflammation
Abstract
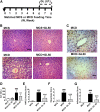
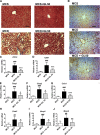
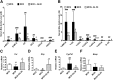
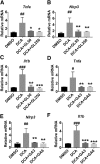
Nonalcoholic steatohepatitis (NASH) is the progressive stage of nonalcoholic fatty liver disease that may ultimately lead to cirrhosis and liver cancer, and there are few therapeutic options for its treatment. Glycyrrhizin (GL), extracted from the traditional Chinese medicine liquorice, has potent hepatoprotective effects in both preclinical animal models and in humans. However, little is currently known about its effects and mechanisms in treating NASH. To explore the effects of GL on NASH, GL or its active metabolite glycyrrhetinic acid (GA) was administered to mice treated with a methionine- and choline-deficient (MCD) diet-induced NASH model, and histologic and biochemical analyses were used to measure the degree of lipid disruption, liver inflammation, and fibrosis. GL significantly improved MCD diet-induced hepatic steatosis, inflammation, and fibrosis and inhibited activation of the NLR family pyrin domain-containing 3 (NLRP3) inflammasome. GL significantly attenuated serum bile acid accumulation in MCD diet-fed mice partially by restoring inflammation-mediated hepatic farnesoid X receptor inhibition. In Raw 264.7 macrophage cells, both GL and GA inhibited deoxycholic acid-induced NLRP3 inflammasome-associated inflammation. Notably, both intraperitoneal injection of GL's active metabolite GA and oral administration of GL prevented NASH in mice, indicating that GL may attenuate NASH via its active metabolite GA. These results reveal that GL, via restoration of bile acid homeostasis and inhibition of inflammatory injury, can be a therapeutic option for treatment of NASH.
U.S. Government work not protected by U.S. copyright.
Figures



References
-
- Akao T, Hayashi T, Kobashi K, Kanaoka M, Kato H, Kobayashi M, Takeda S, Oyama T. (1994) Intestinal bacterial hydrolysis is indispensable to absorption of 18 beta-glycyrrhetic acid after oral administration of glycyrrhizin in rats. J Pharm Pharmacol 46:135–137. - PubMed
-
- Bechmann LP, Kocabayoglu P, Sowa JP, Sydor S, Best J, Schlattjan M, Beilfuss A, Schmitt J, Hannivoort RA, Kilicarslan A, et al. (2013) Free fatty acids repress small heterodimer partner (SHP) activation and adiponectin counteracts bile acid-induced liver injury in superobese patients with nonalcoholic steatohepatitis. Hepatology 57:1394–1406. - PubMed
-
- Chaurasia B, Summers SA. (2015) Ceramides - lipotoxic inducers of metabolic disorders. Trends Endocrinol Metab 26:538–550. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

