Phosphorylation and Signal Transduction Pathways in Translational Control
- PMID: 29959191
- PMCID: PMC6601458
- DOI: 10.1101/cshperspect.a033050
Phosphorylation and Signal Transduction Pathways in Translational Control
Abstract
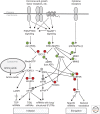
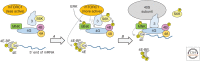
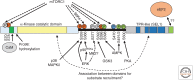
Protein synthesis, including the translation of specific messenger RNAs (mRNAs), is regulated by extracellular stimuli such as hormones and by the levels of certain nutrients within cells. This control involves several well-understood signaling pathways and protein kinases, which regulate the phosphorylation of proteins that control the translational machinery. These pathways include the mechanistic target of rapamycin complex 1 (mTORC1), its downstream effectors, and the mitogen-activated protein (MAP) kinase (extracellular ligand-regulated kinase [ERK]) signaling pathway. This review describes the regulatory mechanisms that control translation initiation and elongation factors, in particular the effects of phosphorylation on their interactions or activities. It also discusses current knowledge concerning the impact of these control systems on the translation of specific mRNAs or subsets of mRNAs, both in physiological processes and in diseases such as cancer.
Copyright © 2019 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
References
-
- Beggs JE, Tian S, Jones GG, Xie J, Iadevaia V, Jenei V, Thomas GJ, Proud CG. 2015. The MAP kinase-interacting kinases regulate cell migration, vimentin expression and eIF4E/CYFIP1 binding. Biochem J 467: 63–76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
