Nephrotoxicity of Cancer Immunotherapies: Past, Present and Future
- PMID: 29959196
- PMCID: PMC6065079
- DOI: 10.1681/ASN.2018050488
Nephrotoxicity of Cancer Immunotherapies: Past, Present and Future
Abstract
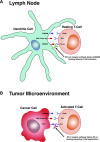
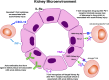
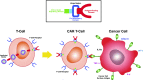
Nephrotoxicity from cancer therapies is common and increasingly encountered in clinical practice, such that the subfield of "onco-nephrology" has emerged. Conventional chemotherapeutic drugs and novel agents targeting specific genes/proteins are effective cancer therapies but suffer from a number of adverse kidney effects. An effective avenue of cancer treatment is immunotherapy, which uses drugs that augment immune system-mediated recognition and targeting of tumor cells. As such, leveraging the immune system to target malignant cells represents an important modality in eradicating cancer. IFN and high-dose IL-2 are older immunotherapies used in clinical practice to treat various malignancies, whereas new cancer immunotherapies have emerged over the past decade that offer even more effective treatment options. The immune checkpoint inhibitors are an exciting addition to the cancer immunotherapy armamentarium. Chimeric antigen receptor T cells are also a new immunotherapy used to treat various hematologic malignancies. However, as with the conventional and targeted cancer agents, the immunotherapies are also associated with immune-related adverse effects, which includes nephrotoxicity.
Keywords: acute kidney injury; chimeric antigen receptor T-cells; immune checkpoint inhibitors; immunotherapies; interferon; interleukin-2.
Copyright © 2018 by the American Society of Nephrology.
Figures

References
-
- Rosner MH, Perazella MA: Acute kidney injury in patients with cancer. N Engl J Med 376: 1770–1781, 2017 - PubMed
-
- Porta C, Cosmai L, Gallieni M, Pedrazzoli P, Malberti F: Renal effects of targeted anticancer therapies. Nat Rev Nephrol 11: 354–370, 2015 - PubMed
-
- Schreiber RD, Old LJ, Smyth MJ: Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 331: 1565–1570, 2011 - PubMed
-
- Hanahan D, Weinberg RA: Hallmarks of cancer: The next generation. Cell 144: 646–674, 2011 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

