Regional Distribution of CNS Antigens Differentially Determines T-Cell Mediated Neuroinflammation in a CX3CR1-Dependent Manner
- PMID: 29959236
- PMCID: PMC6083460
- DOI: 10.1523/JNEUROSCI.0366-18.2018
Regional Distribution of CNS Antigens Differentially Determines T-Cell Mediated Neuroinflammation in a CX3CR1-Dependent Manner
Abstract
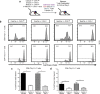
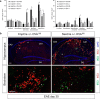
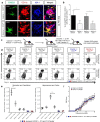
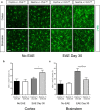
T cells continuously sample CNS-derived antigens in the periphery, yet it is unknown how they sample and respond to CNS antigens derived from distinct brain areas. We expressed ovalbumin (OVA) neoepitopes in regionally distinct CNS areas (Cnp-OVA and Nes-OVA mice) to test peripheral antigen sampling by OVA-specific T cells under homeostatic and neuroinflammatory conditions. We show that antigen sampling in the periphery is independent of regional origin of CNS antigens in both male and female mice. However, experimental autoimmune encephalomyelitis (EAE) is differentially influenced in Cnp-OVA and Nes-OVA female mice. Although there is the same frequency of CD45high CD11b+ CD11c+ CX3CL1+ myeloid cell-T-cell clusters in neoepitope-expressing areas, EAE is inhibited in Nes-OVA female mice and accelerated in CNP-OVA female mice. Accumulation of OVA-specific T cells and their immunomodulatory effects on EAE are CX3C chemokine receptor 1 (CX3CR1) dependent. These data show that despite similar levels of peripheral antigen sampling, CNS antigen-specific T cells differentially influence neuroinflammatory disease depending on the location of cognate antigens and the presence of CX3CL1/CX3CR1 signaling.SIGNIFICANCE STATEMENT Our data show that peripheral T cells similarly recognize neoepitopes independent of their origin within the CNS under homeostatic conditions. Contrastingly, during ongoing autoimmune neuroinflammation, neoepitope-specific T cells differentially influence clinical score and pathology based on the CNS regional location of the neoepitopes in a CX3CR1-dependent manner. Altogether, we propose a novel mechanism for how T cells respond to regionally distinct CNS derived antigens and contribute to CNS autoimmune pathology.
Keywords: CNS; T cells; autoimmunity; neuroinflammation.
Copyright © 2018 the authors 0270-6474/18/387058-14$15.00/0.
Figures



References
-
- Anandasabapathy N, Victora GD, Meredith M, Feder R, Dong B, Kluger C, Yao K, Dustin ML, Nussenzweig MC, Steinman RM, Liu K (2011) Flt3L controls the development of radiosensitive dendritic cells in the meninges and choroid plexus of the steady-state mouse brain. J Exp Med 208:1695–1705. 10.1084/jem.20102657 - DOI - PMC - PubMed
-
- Bonfiglio T, Olivero G, Merega E, Di Prisco S, Padolecchia C, Grilli M, Milanese M, Di Cesare Mannelli L, Ghelardini C, Bonanno G, Marchi M, Pittaluga A (2017) Prophylactic versus therapeutic fingolimod: restoration of presynaptic defects in mice suffering from experimental autoimmune encephalomyelitis. PLoS One 12:e0170825. 10.1371/journal.pone.0170825 - DOI - PMC - PubMed
-
- Böttcher JP, Beyer M, Meissner F, Abdullah Z, Sander J, Höchst B, Eickhoff S, Rieckmann JC, Russo C, Bauer T, Flecken T, Giesen D, Engel D, Jung S, Busch DH, Protzer U, Thimme R, Mann M, Kurts C, Schultze JL, et al. (2015) Functional classification of memory CD8(+) T cells by CX3CR1 expression. Nat Commun 6:8306. 10.1038/ncomms9306 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
