The Hsp70 inhibitor 2-phenylethynesulfonamide inhibits replication and carcinogenicity of Epstein-Barr virus by inhibiting the molecular chaperone function of Hsp70
- PMID: 29959331
- PMCID: PMC6026193
- DOI: 10.1038/s41419-018-0779-3
The Hsp70 inhibitor 2-phenylethynesulfonamide inhibits replication and carcinogenicity of Epstein-Barr virus by inhibiting the molecular chaperone function of Hsp70
Abstract
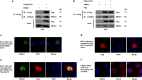
Epstein-Barr virus (EBV) can infect cells in latent and lytic period and cause serious disease. Epstein-Barr virus nuclear antigen 1 (EBNA1) is essential for the maintenance of the EBV DNA episome, replication and transcription. 2-phenylethynesulfonamide (PES) is a small molecular inhibitor of Heat shock protein 70 (Hsp70), which can interact with Hsp70 and disrupts its association with co-chaperones and substrate proteins of Hsp70. In our study, we found that PES could decrease the expression of EBNA1, which is independent of effects on EBNA1 transcription or proteasomal degradation pathway. The central glycine-alanine repeats domain was not required for inhibition of EBNA1 expression by PES. Also, PES could reduce the amount of intracellular EBV genomic DNA. PES inhibited proliferation and migration but induced cell cycle arrest and apoptosis of EBV positive cells. In addition, silencing of Hsp70 decreased expression of EBNA1 and the amounts of intracellular EBV genomic DNA, and PES increased this effect on a dose-dependent manner. On the contrast, over-expression of Hsp70 enhanced the expression of EBNA1 and the amounts of intracellular EBV genomic DNA, but PES inhibited this effect on a dose-dependent manner. Furthermore, Hsp70 interacted with EBNA1 but PES interfered this interaction. Our results indicate that PES suppresses replication and carcinogenicity of Epstein-Barr virus via inhibiting the molecular chaperone function of Hsp70.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures






References
-
- Raab-Traub N. Epstein–Barr virus and nasopharyngeal carcinoma. Semin. Cancer Biol. 1992;3:297–307. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 31270205/National Natural Science Foundation of China (National Science Foundation of China)/International
- 31270205/National Natural Science Foundation of China (National Science Foundation of China)/International
- 31270205/National Natural Science Foundation of China (National Science Foundation of China)/International
- 31270205/National Natural Science Foundation of China (National Science Foundation of China)/International
- 31270205/National Natural Science Foundation of China (National Science Foundation of China)/International
LinkOut - more resources
Full Text Sources
Other Literature Sources

