The pial vasculature of the mouse develops according to a sensory-independent program
- PMID: 29959346
- PMCID: PMC6026131
- DOI: 10.1038/s41598-018-27910-3
The pial vasculature of the mouse develops according to a sensory-independent program
Abstract
The cerebral vasculature is organized to supply the brain's metabolic needs. Sensory deprivation during the early postnatal period causes altered neural activity and lower metabolic demand. Neural activity is instructional for some aspects of vascular development, and deprivation causes changes in capillary density in the deprived brain region. However, it is not known if the pial arteriole network, which contains many leptomeningeal anastomoses (LMAs) that endow the network with redundancy against occlusions, is also affected by sensory deprivation. We quantified the effects of early-life sensory deprivation via whisker plucking on the densities of LMAs and penetrating arterioles (PAs) in anatomically-identified primary sensory regions (vibrissae cortex, forelimb/hindlimb cortex, visual cortex and auditory cortex) in mice. We found that the densities of penetrating arterioles were the same across cortical regions, though the hindlimb representation had a higher density of LMAs than other sensory regions. We found that the densities of PAs and LMAs, as well as quantitative measures of network topology, were not affected by sensory deprivation. Our results show that the postnatal development of the pial arterial network is robust to sensory deprivation.
Conflict of interest statement
The authors declare no competing interests.
Figures


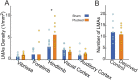


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

