Safety and effectiveness of eculizumab for adult patients with atypical hemolytic-uremic syndrome in Japan: interim analysis of post-marketing surveillance
- PMID: 29959568
- PMCID: PMC6344388
- DOI: 10.1007/s10157-018-1609-8
Safety and effectiveness of eculizumab for adult patients with atypical hemolytic-uremic syndrome in Japan: interim analysis of post-marketing surveillance
Abstract
Background: Eculizumab has been available for the treatment of atypical hemolytic-uremic syndrome (aHUS) in Japan since 2013. To assess safety and effectiveness of eculizumab in adult aHUS patients in the real-life setting, we performed interim analysis of a post-marketing surveillance mandated by Japanese regulations.
Methods: This study enrolled any patient who was diagnosed with TMA excluding Shiga toxin-producing Escherichia coli-HUS or thrombotic thrombocytopenic purpura based on Japanese clinical guide published in 2013 as inclusion criteria and treated with eculizumab. Although the term aHUS was redefined to denote only complement-mediated HUS in the guide revised in 2016, the patients with TMA caused by other causes (secondary TMA) were included. Patient outcomes and safety were evaluated at 6 months, 12 months, and annually thereafter.
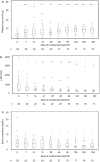
Results: Thirty-three patients with aHUS and 27 patients with secondary TMA were enrolled. Median treatment duration of aHUS was 24weeks. Complement genes variants were detected in 11 of 18 patients with aHUS (61.1%). Among the 29 aHUS patients with available baseline data, platelet count (PLT), lactic dehydrogenase and serum creatinine (SCr) improved within 1-month after eculizumab initiation. TMA event-free status, complete TMA response, PLT normalization, and SCr decrease were achieved in 67.9% (19/28), 27.8% (5/18), 56.5% (13/23), and 57.1% (16/28) of patients, respectively. Thirty-three and 11 adverse reactions were observed in patients with aHUS (13/33 patients) and secondary TMA (6/27 patients), respectively.
Conclusions: This interim analysis confirmed the acceptable safety profile and effectiveness of eculizumab for Japanese adult aHUS patients in real-world settings.
Keywords: Atypical hemolytic–uremic syndrome; C5 inhibitor; Complement; Eculizumab; Post-marketing surveillance.
Conflict of interest statement
Conflict of interest
Employment: Takahisa Matsuda and Akihiko Shimono (Alexion Pharma GK), Advisory role: Yoshitaka Miyakawa (Ablynx, Chugai Pharmaceutical Co., Ltd., FUJIFILM Pharma Co., Ltd, Novartis Pharmaceuticals and Zenyaku Kogyo Co., Ltd.), Stock ownership: Yoshitaka Miyakawa (Preventive Medicine), Patent royalties: Yoshihiro Fujimura (Alfresa Pharma Corporation), Honoraria: Yoshihiko Hidaka, Norimitsu Inoue, Shuichi Ito, Shinya Kaname, Hideki Kato and Masaomi Nangaku (Alexion Pharma GK), Masanori Matsumoto (Alexion Pharma GK and Asahi Kasei Pharma Corporation), Hirokazu Okada (Alexion Pharma GK and Kyowa Hakko Kirin Co. Ltd.), Yoshitaka Miyakawa (Alexion Pharma GK, Bayer Yakuhin, Ltd., Baxalta Japan Limited, Bioverativ, Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Company Limited, Kyowa Hakko Kirin Co. Ltd., Novartis Pharmaceuticals, Teijin Pharma Limited and Zenyaku Kogyo Co., Ltd.), Shoichi Maruyama (Alexion Pharma GK, Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Company Limited, Kyowa Hakko Kirin Co. Ltd., Mochida Pharmaceutical Co. Ltd., Otsuka Pharmaceutical Co., Ltd., Sanwa Kagaku Kenkyusho Co., Ltd., and Sumitomo Dainippon Pharma Co., Ltd.), Research funding: Shoichi Maruyama (Sanwa Kagaku Kenkyusho Co., Ltd.) and Masanori Matsumoto (Bayer Yakuhin, Ltd., Baxalta Japan Limited and Chugai Pharmaceutical Co., Ltd.), Subsidies: Masaomi Nangaku (Alexion Pharma GK), Shuichi Ito (Astellas Pharma Inc., Chugai Pharmaceutical Co., Ltd. and Pfizer Japan Inc.), Shoichi Maruyama (Alexion Pharma GK, Asahi Kasei Pharma Corporation, Astellas Pharma Inc., Baxter, Bristol-Myers Squibb, Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Company Limited, Kissei Pharmaceutical Co., Ltd., Kyowa Hakko Kirin Co. Ltd., Mitsubishi Tanabe Pharma, Mochida Pharmaceutical Co. Ltd., MSD K.K., Nippon Boehringer Ingelheim Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Pfizer Japan Inc., ROHTO Pharmaceutical Co.,Ltd., Sanwa Kagaku Kenkyusho Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Takeda Pharmaceutical Company Limited, Teijin Pharma Limited and Torii Pharmaceutical Co.,Ltd) and Hirokazu Okada (Astellas Pharma Inc., Ltd., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Company Limited., Kyowa Hakko Kirin Co. Ltd., Mitsubishi Tanabe Pharma, MSD K.K., Nippon Boehringer Ingelheim Co., Novartis Pharmaceuticals, Otsuka Pharmaceutical Co., Ltd., Pfizer Japan Inc., Shionogi & Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Takeda Pharmaceutical Company Limited and Torii Pharmaceutical Co.,Ltd.), Endowed departments by commercial entities: Masashi Mizuno (Baxter Japan).
Figures
References
-
- Laurence J, Haller H, Mannucci PM, Nangaku M, Praga M, Rodriguez de Cordoba S. Atypical hemolytic uremic syndrome (aHUS): essential aspects of an accurate diagnosis. Clin Adv Hematol Oncol. 2016;14(Suppl 11):2–15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

