Disruption of MEK/ERK/c-Myc signaling radiosensitizes prostate cancer cells in vitro and in vivo
- PMID: 29959569
- PMCID: PMC11813462
- DOI: 10.1007/s00432-018-2696-3
Disruption of MEK/ERK/c-Myc signaling radiosensitizes prostate cancer cells in vitro and in vivo
Abstract
Purpose: Prostate cancer (PCa) cell radioresistance causes the failure of radiation therapy (RT) in localized or locally advanced disease. The aberrant accumulation of c-Myc oncoprotein, known to promote PCa onset and progression, may be due to the control of gene transcription and/or MEK/ERK-regulated protein stabilization. Here, we investigated the role of MEK/ERK signaling in PCa.
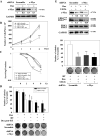
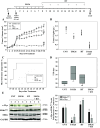
Methods: LnCAP, 22Rv1, DU145, and PC3 PCa cell lines were used in in vitro and in vivo experiments. U0126, trametinib MEK/ERK inhibitors, and c-Myc shRNAs were used. Radiation was delivered using an x-6 MV photon linear accelerator. U0126 in vivo activity alone or in combination with irradiation was determined in murine xenografts.
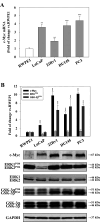
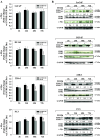
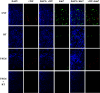
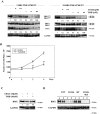
Results: Inhibition of MEK/ERK signaling down-regulated c-Myc protein in PCa cell lines to varying extents by affecting expression of RNA and protein, which in turn determined radiosensitization in in vitro and in vivo xenograft models of PCa cells. The crucial role played by c-Myc in the MEK/ERK pathways was demonstrated in 22Rv1 cells by the silencing of c-Myc by means of short hairpin mRNA, which yielded effects resembling the targeting of MEK/ERK signaling. The clinically approved compound trametinib used in vitro yielded the same effects as U0126 on growth and C-Myc expression. Notably, U0126 and trametinib induced a drastic down-regulation of BMX, which is known to prevent apoptosis in cancer cells.
Conclusions: The results of our study suggest that signal transduction-based therapy can, by disrupting the MEK/ERK/c-Myc axis, reduce human PCa radioresistance caused by increased c-Myc expression in vivo and in vitro and restores apoptosis signals.
Keywords: C-Myc; ERKs; Prostate cancer; Radioresistance; Radiotherapy; U0126.
Conflict of interest statement
The authors have no affiliations to disclose, including any of a financial nature, that they consider to be relevant or related to the subject matter discussed.
Figures

References
-
- Bethel CR, Faith D, Li X, Guan B, Hicks JL, Lan F, Jenkins RB, Bieberich CJ, De Marzo AM (2006) Decreased NKX3.1 protein expression in focal prostatic atrophy, prostatic intraepithelial neoplasia, and adenocarcinoma: association with gleason score and chromosome 8p deletion. Cancer Res 66(22):10683–10690 - DOI - PubMed
-
- Bretones G, Delgado MD, León J (2015) C-Myc and cell cycle control. BBA 1849:506–516 - PubMed
-
- Ciccarelli C, Vulcano F, Milazzo L, Gravina GL, Marampon F, Macioce G, Giampaolo A, Tombolini V, Di Paolo V, Hassan HJ, Zani BM (2016) Key role of MEK/ERK pathway in sustaining tumorigenicity and in vitro radioresistance of embryonal rhabdomyosarcoma stem-like cell population. Mol Cancer 15:16 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

