Atraumatic splenic rupture in a peritoneal dialysis patient
- PMID: 29959617
- PMCID: PMC6181893
- DOI: 10.1007/s13730-018-0346-x
Atraumatic splenic rupture in a peritoneal dialysis patient
Abstract
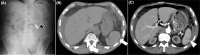
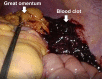
Splenic hemorrhage is a potentially life-threatening complication usually occurring after blunt trauma to the abdomen. Atraumatic splenic rupture (ASR) is an uncommon condition, and mostly results from pathology affecting the spleen, such as tumor infiltration or infection. Here, we report a case of atraumatic rupture of a normal spleen in a patient undergoing peritoneal dialysis, and review similar cases in the literature. The case involved a 58-year-old man with nephrotic syndrome who had been undergoing peritoneal dialysis for 1 year. He presented to the hospital with abdominal pain, nausea, and blood-stained dialysate. Laboratory data revealed severe anemia, with a hemoglobin of 4.3 g/dL. An abdominal computed tomography (CT) scan demonstrated a high-density area around the spleen and malposition of the catheter. Laparoscopy revealed large amounts of coagulated blood surrounding the spleen. The patient was diagnosed with atraumatic splenic bleeding. He improved with bed rest and blood transfusion, and could continue with peritoneal dialysis. It was considered that the etiology of bleeding was directly from the spleen. However, due to the temporary malposition of the peritoneal catheter, catheter-induced splenic trauma could not be ruled out. ASR is a rare entity that needs a high index of suspicion for diagnosis. Using CT scanning and peritoneal fluid analysis, these modalities may assist in the diagnosis. Emergency intervention is required upon definitive diagnosis. Increased awareness of ASR can enhance the early diagnosis and effective treatment.
Keywords: Atraumatic splenic rupture; Chronic renal failure; Peritoneal dialysis.
Conflict of interest statement
Conflict of interest
The authors have declared that no conflict of interest exists.
Human and animal rights statement
This article does not describe any studies with human participants performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

