cGMP mediates short- and long-term modulation of excitability in a decision-making neuron in Aplysia
- PMID: 29960055
- PMCID: PMC6174080
- DOI: 10.1016/j.neulet.2018.06.046
cGMP mediates short- and long-term modulation of excitability in a decision-making neuron in Aplysia
Abstract
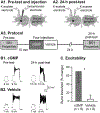
In elementary neural circuits, changes in excitability can have a strong impact in the expression of a given behavior. One example is provided by B51, a neuron with decision-making properties in the feeding neural circuit of the mollusk Aplysia. The excitability of B51 is bidirectionally modulated by external and internal stimuli in a manner that is consistent with the corresponding induced changes in feeding behavior. For example, in operant reward learning, which up-regulates feeding, B51 excitability is increased via a cAMP-dependent mechanism. Conversely, following training protocols with aversive stimuli, which down-regulate feeding, B51 excitability is decreased. In this study, we tested the hypothesis that B51 decreased excitability may be mediated by another cyclic nucleotide, cGMP. Our results revealed that iontophoretic injection of cGMP was capable of inducing both short-term (45 min) and long-term (24 h) reduction of B51 excitability. We next investigated which biochemical trigger could increase cGMP cytosolic levels. The neurotransmitter nitric oxide was found to decrease B51 excitability through the activation of the soluble guanylyl cyclase. These findings indicate that a cGMP-dependent pathway modulates B51 excitability in a manner opposite of cAMP, indicating that distinct cyclic-nucleotide pathways bidirectionally regulate the excitability of a decision-making neuron.
Keywords: Aplysia; Excitability; Neuronal plasticity; Second messengers.
Copyright © 2018 Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of Interest
The authors have no actual or potential conflicts of interest.
Figures




Similar articles
-
Critical role of protein kinase G in the long-term balance between defensive and appetitive behaviors induced by aversive stimuli in Aplysia.Behav Brain Res. 2020 Apr 6;383:112504. doi: 10.1016/j.bbr.2020.112504. Epub 2020 Jan 22. Behav Brain Res. 2020. PMID: 31981653 Free PMC article.
-
Change in excitability of a putative decision-making neuron in Aplysia serves as a mechanism in the decision not to feed following food satiation.Behav Brain Res. 2015 Mar 15;281:131-6. doi: 10.1016/j.bbr.2014.12.022. Epub 2014 Dec 16. Behav Brain Res. 2015. PMID: 25527117 Free PMC article.
-
Specific Plasticity Loci and Their Synergism Mediate Operant Conditioning.J Neurosci. 2022 Feb 16;42(7):1211-1223. doi: 10.1523/JNEUROSCI.1722-21.2021. Epub 2022 Jan 6. J Neurosci. 2022. PMID: 34992131 Free PMC article.
-
Feeding behavior of Aplysia: a model system for comparing cellular mechanisms of classical and operant conditioning.Learn Mem. 2006 Nov-Dec;13(6):669-80. doi: 10.1101/lm.339206. Learn Mem. 2006. PMID: 17142299 Review.
-
cGMP and S-nitrosylation: two routes for modulation of neuronal excitability by NO.Trends Neurosci. 2002 Oct;25(10):510-7. doi: 10.1016/s0166-2236(02)02254-3. Trends Neurosci. 2002. PMID: 12220879 Review.
Cited by
-
Nitrergic modulation of ion channel function in regulating neuronal excitability.Channels (Austin). 2021 Dec;15(1):666-679. doi: 10.1080/19336950.2021.2002594. Channels (Austin). 2021. PMID: 34802368 Free PMC article.
-
Critical role of protein kinase G in the long-term balance between defensive and appetitive behaviors induced by aversive stimuli in Aplysia.Behav Brain Res. 2020 Apr 6;383:112504. doi: 10.1016/j.bbr.2020.112504. Epub 2020 Jan 22. Behav Brain Res. 2020. PMID: 31981653 Free PMC article.
-
Role of nitric oxide in the induction of the behavioral and cellular changes produced by a common aversive stimulus in Aplysia.Behav Brain Res. 2019 Mar 15;360:341-353. doi: 10.1016/j.bbr.2018.12.010. Epub 2018 Dec 6. Behav Brain Res. 2019. PMID: 30528940 Free PMC article.
-
Persistent modulatory actions and task switching in the feeding network of Aplysia.Curr Opin Neurobiol. 2023 Oct;82:102775. doi: 10.1016/j.conb.2023.102775. Epub 2023 Aug 23. Curr Opin Neurobiol. 2023. PMID: 37625344 Free PMC article. Review.
-
Role of serotonin in the lack of sensitization caused by prolonged food deprivation in Aplysia.Behav Brain Res. 2024 Feb 26;458:114736. doi: 10.1016/j.bbr.2023.114736. Epub 2023 Nov 1. Behav Brain Res. 2024. PMID: 37923220 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

