Frequent Adverse Drug Reactions, and Medication Groups under Suspicion
- PMID: 29960607
- PMCID: PMC6041966
- DOI: 10.3238/arztebl.2018.0393
Frequent Adverse Drug Reactions, and Medication Groups under Suspicion
Abstract
Background: The adverse drug reaction database of the German Federal Institute for Drugs and Medical Devices (Bundesinstitut für Arzneimittel und Medizinprodukte, BfArM) contains reports of suspected adverse drug reactions (ADRs) that are spon- taneously submitted by physicians, pharmacists, or patients. The aim of the present study was a descriptive analysis of all of these spontaneous reports.
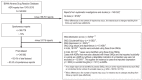
Methods: 345 662 spontaneously submitted reports were analyzed with respect to the number of reports per year, the sources of the reports, demographic variables, the most commonly reported ADRs, and the drug classes most commonly suspected.
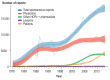
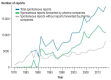
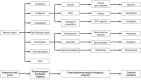
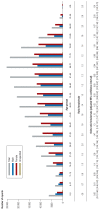
Results: The number of reports submitted spontaneously each year has grown steadily since 1978. At the least detailed level of analysis, "drugs for the treatment of nervous system disorders" were the most common class of drugs under suspicion of causing the reported adverse drug reactions (23.1%). In a more detailed analysis by therapeutic subgroup, the three subgroups most commonly reported as suspected of causing side effects were antithrombotic agents, systemic antibiotics, and psycholeptics-causing thrombocytopenia, diarrhea, and drug dependency as the most frequently reported ADRs, respectively. The order of drug classes most commonly causing ADRs differed markedly between the physicians' reports (diazepines, fluoroquinolones, heparins) and the patients' reports (interferons, anti- thrombotic drugs, selective immunosuppressant drugs). Patients more commonly reported subjectively perceived ADRs, while physicians more commonly reported findings or diagnoses that require medical expertise.
Conclusion: The increasing number of spontaneous reports is mainly due to reports forwarded from pharmaceutical companies to the BfArM. This, in turn, is probably a result of increasingly strict legal reporting requirements in Germany. The detected differences between physicians' and patients' ADR reports can be taken to indicate that patients should be more specifically informed and questioned about potential ADRs. By reporting adverse drug reactions, physicians may improve drug safety.
Figures

Comment in
-
Unanswered Questions.Dtsch Arztebl Int. 2018 Oct 12;115(41):682. doi: 10.3238/arztebl.2018.0682a. Dtsch Arztebl Int. 2018. PMID: 30406747 Free PMC article. No abstract available.
References
-
- Coloma PM, Trifirò G, Patadia V, Sturkenboom M. Postmarketing safety surveillance: where does signal detection using electronic healthcare records fit into the big picture? Drug Saf. 2013;36:183–197. - PubMed
-
- Paludetto MN, Olivier-Abbal P, Montastruc JL. Is spontaneous reporting always the most important information supporting drug withdrawals for pharmacovigilance reasons in France? Pharmacoepidemiol Drug Saf. 2012;21:1289–1294. - PubMed
-
- Amery WK. Why there is a need for pharmacovigilance. Pharmacoepidemiol Drug Saf. 1999;8:61–64. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

