The artificial placenta: Continued lung development during extracorporeal support in a preterm lamb model
- PMID: 29960740
- PMCID: PMC6151273
- DOI: 10.1016/j.jpedsurg.2018.06.001
The artificial placenta: Continued lung development during extracorporeal support in a preterm lamb model
Abstract
Purpose: An artificial placenta (AP) utilizing extracorporeal life support (ECLS) could avoid the harm of mechanical ventilation (MV) while allowing the lungs to develop.
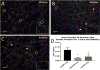
Methods: AP lambs (n = 5) were delivered at 118 days gestational age (GA; term = 145 days) and placed on venovenous ECLS (VV-ECLS) with jugular drainage and umbilical vein reinfusion. Lungs remained fluid-filled. After 10 days, lambs were ventilated. MV control lambs were delivered at 118 ("early MV"; n = 5) or 128 days ("late MV"; n = 5), and ventilated. Compliance and oxygenation index (OI) were calculated. After sacrifice, lungs were procured and H&E-stained slides scored for lung injury. Slides were also immunostained for PDGFR-α and α-actin; alveolar development was quantified by the area fraction of alveolar septal tips staining double-positive for both markers.
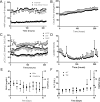
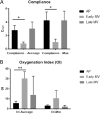
Results: Compliance of AP lambs was 2.79 ± 0.81 Cdyn compared to 0.83 ± 0.19 and 3.04 ± 0.99 for early and late MV, respectively. OI in AP lambs was lower than early MV lambs (6.20 ± 2.10 vs. 36.8 ± 16.8) and lung injury lower as well (1.8 ± 1.6 vs. 6.0 ± 1.2). Double-positive area fractions were higher in AP lambs (0.012 ± 0.003) than early (0.003 ± 0.0005) and late (0.004 ± 0.002) MV controls.
Conclusions: Lung development continues and lungs are protected from injury during AP support relative to mechanical ventilation.
Level of evidence: n/a (basic/translational science).
Keywords: Artificial placenta; Extracorporeal life support; Lung development; Prematurity.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures


Comment in
-
Reply to Letter to the Editor: The Artificial Womb: What is the Next Step?J Pediatr Surg. 2019 Feb;54(2):363. doi: 10.1016/j.jpedsurg.2018.10.069. Epub 2018 Nov 6. J Pediatr Surg. 2019. PMID: 30497822 No abstract available.
-
The artificial womb: What is the next step?J Pediatr Surg. 2019 Feb;54(2):362. doi: 10.1016/j.jpedsurg.2018.08.063. Epub 2018 Nov 5. J Pediatr Surg. 2019. PMID: 30611526 No abstract available.
Similar articles
-
An Artificial Placenta Protects Against Lung Injury and Promotes Continued Lung Development in Extremely Premature Lambs.ASAIO J. 2019 Sep/Oct;65(7):690-697. doi: 10.1097/MAT.0000000000000939. ASAIO J. 2019. PMID: 30585874 Free PMC article.
-
Perfluorocarbons Prevent Lung Injury and Promote Development during Artificial Placenta Support in Extremely Premature Lambs.Neonatology. 2018;113(4):313-321. doi: 10.1159/000486387. Epub 2018 Feb 23. Neonatology. 2018. PMID: 29478055 Free PMC article.
-
Effects of an artificial placenta on brain development and injury in premature lambs.J Pediatr Surg. 2018 Jun;53(6):1234-1239. doi: 10.1016/j.jpedsurg.2018.02.091. Epub 2018 Mar 8. J Pediatr Surg. 2018. PMID: 29605267 Free PMC article.
-
Artificial placenta: Analysis of recent progress.Eur J Obstet Gynecol Reprod Biol. 2017 Jan;208:61-70. doi: 10.1016/j.ejogrb.2016.11.005. Epub 2016 Nov 11. Eur J Obstet Gynecol Reprod Biol. 2017. PMID: 27894031 Review.
-
A paradigm shift in the treatment of extreme prematurity: the artificial placenta.Curr Opin Pediatr. 2014 Jun;26(3):370-6. doi: 10.1097/MOP.0000000000000083. Curr Opin Pediatr. 2014. PMID: 24786370 Free PMC article. Review.
Cited by
-
The artificial placenta and EXTEND technologies: one of these things is not like the other.J Perinatol. 2023 Nov;43(11):1343-1348. doi: 10.1038/s41372-023-01716-2. Epub 2023 Jul 1. J Perinatol. 2023. PMID: 37393398
-
Advances in extracorporeal membrane oxygenator design for artificial placenta technology.Artif Organs. 2021 Mar;45(3):205-221. doi: 10.1111/aor.13827. Epub 2020 Nov 4. Artif Organs. 2021. PMID: 32979857 Free PMC article. Review.
-
Achieving sustained extrauterine life: Challenges of an artificial placenta in fetal pigs as a model of the preterm human fetus.Physiol Rep. 2021 Mar;9(5):e14742. doi: 10.14814/phy2.14742. Physiol Rep. 2021. PMID: 33650787 Free PMC article.
-
A Pumpless Microfluidic Neonatal Lung Assist Device for Support of Preterm Neonates in Respiratory Distress.Adv Sci (Weinh). 2020 Sep 29;7(21):2001860. doi: 10.1002/advs.202001860. eCollection 2020 Nov. Adv Sci (Weinh). 2020. PMID: 33173732 Free PMC article.
-
Gestational Age Influences the Early Microarchitectural Changes in Response to Mechanical Ventilation in the Preterm Lamb Lung.Front Pediatr. 2019 Aug 21;7:325. doi: 10.3389/fped.2019.00325. eCollection 2019. Front Pediatr. 2019. PMID: 31497582 Free PMC article.
References
-
- National Prematurity Awareness Month. Centers for Disease Control and Prevention. 2015
-
- Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet (London, England) 2012;379:2162–2172. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

