Continuous ST-Monitoring Function of Implantable Cardioverter Defibrillator Detects Silent Ischemia in Patients With Coronary Artery Disease
- PMID: 29960992
- PMCID: PMC6064887
- DOI: 10.1161/JAHA.118.009332
Continuous ST-Monitoring Function of Implantable Cardioverter Defibrillator Detects Silent Ischemia in Patients With Coronary Artery Disease
Abstract
Background: Newer implantable cardioverter defibrillators can monitor intracardiac ECGs , but their ability to detect ischemia is unclear. This study investigated the usefulness of implantable cardioverter defibrillators with an ST-monitoring function in coronary artery disease patients.
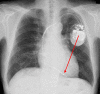
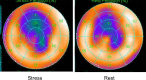
Methods and results: We conducted a prospective study of implantable cardioverter defibrillator patients with the ST-monitoring function. One hundred seventy-three patients who received implantable cardioverter defibrillators for primary or secondary prevention of sudden cardiac death. All patients underwent medical examinations at least every 6 months, with standard 12-lead ECGs and device checks that included analysis of the ST-monitoring function. Myocardial perfusion imaging or coronary angiography was performed during the follow-up. The mean follow-up duration was 23.3±7.7 months. Significant ST changes occurred in 15 patients (8.7%), of whom 14 were asymptomatic. The incidence of angina pectoris was significantly higher in the ST change (+) group than that in the ST change (-) group (28.6% versus 7.2%, P=0.03). In the patients who underwent myocardial perfusion imaging, the sensitivity, specificity, and negative predictive value of the ST-monitoring feature to detect ischemia were 75.0%, 72.5%, and 93.5%, respectively. The sensitivity, specificity, and negative predictive value of the ST-monitoring feature to predict residual stenosis evaluated using coronary angiography were 76.9%, 83.5%, and 97.5%, respectively. The percentage of patients with a septal right ventricular lead was significantly lower in the ST change (+) group than in the ST change (-) group (13.5% versus 33.5%, P=0.01).
Conclusions: If intracardiac ECGs ST changes are detected, it is necessary to use additional modalities even in asymptomatic patients.
Clinical trial registration: URL: upload.umin.ac.jp. Unique identifier: UMIN000011824.
Keywords: coronary artery disease; implantable cardioverter‐defibrillator; ischemia.
© 2018 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley.
Figures


Similar articles
-
Evaluation of the Impact of a Chronic Total Coronary Occlusion on Ventricular Arrhythmias and Long-Term Mortality in Patients With Ischemic Cardiomyopathy and an Implantable Cardioverter-Defibrillator (the eCTOpy-in-ICD Study).J Am Heart Assoc. 2018 May 2;7(10):e008609. doi: 10.1161/JAHA.118.008609. J Am Heart Assoc. 2018. PMID: 29720502 Free PMC article.
-
Protein biomarkers identify patients unlikely to benefit from primary prevention implantable cardioverter defibrillators: findings from the Prospective Observational Study of Implantable Cardioverter Defibrillators (PROSE-ICD).Circ Arrhythm Electrophysiol. 2014 Dec;7(6):1084-91. doi: 10.1161/CIRCEP.113.001705. Epub 2014 Oct 1. Circ Arrhythm Electrophysiol. 2014. PMID: 25273351 Free PMC article.
-
Implantable cardioverter-defibrillator therapy among patients with non-ischaemic vs. ischaemic cardiomyopathy for primary prevention of sudden cardiac death.Europace. 2018 Jan 1;20(1):65-72. doi: 10.1093/europace/euw379. Europace. 2018. PMID: 28082419
-
Implantable cardioverter-defibrillators: indications and unresolved issues.Tex Heart Inst J. 2012;39(3):335-41. Tex Heart Inst J. 2012. PMID: 22719141 Free PMC article. Review.
-
Subcutaneous implantable cardioverter defibrillator indication in prevention of sudden cardiac death in difficult clinical situations: A French expert position paper.Arch Cardiovasc Dis. 2020 May;113(5):359-366. doi: 10.1016/j.acvd.2020.03.011. Epub 2020 Apr 22. Arch Cardiovasc Dis. 2020. PMID: 32334981 Review.
Cited by
-
The AngelMed Guardian® System in the Detection of Coronary Artery Occlusion: Current Perspectives.Med Devices (Auckl). 2020 Jan 7;13:1-12. doi: 10.2147/MDER.S219865. eCollection 2020. Med Devices (Auckl). 2020. PMID: 32021496 Free PMC article. Review.
-
Clinical valuation of ST changes in a group of patients with ventricular arrhythmias: The inSighT Study.Ann Noninvasive Electrocardiol. 2022 May;27(3):e12914. doi: 10.1111/anec.12914. Epub 2022 Feb 15. Ann Noninvasive Electrocardiol. 2022. PMID: 35170151 Free PMC article.
References
-
- Menees DS, Peterson ED, Wang Y, Curtis JP, Messenger JC, Rumsfeld JS, Gurm HS. Door‐to‐balloon time and mortality among patients undergoing primary PCI. N Engl J Med. 2013;369:901–909. - PubMed
-
- Myerburg RJ, Interian A Jr, Mitrani RM, Kessler KM, Castellanos A. Frequency of sudden cardiac death and profiles of risk. Am J Cardiol. 1997;80:10F–19F. - PubMed
-
- Cannom DS, Prystowsky EN. Management of ventricular arrhythmias: detection, drugs, and devices. JAMA. 1999;281:172–179. - PubMed
-
- Daubert JP, Zareba W, Cannom DS, McNitt S, Rosero SZ, Wang P, Schuger C, Steinberg JS, Higgins SL, Wilber DJ, Klein H, Andrews ML, Hall WJ, Moss AJ; MADIT II Investigators . Inappropriate implantable cardioverter‐defibrillator shocks in MADIT II: frequency, mechanisms, predictors, and survival impact. J Am Coll Cardiol. 2008;51:1357–1365. - PubMed
-
- Packer DL, Prutkin JM, Hellkamp AS, Mitchell LB, Bernstein RC, Wood F, Boehmer JP, Carlson MD, Frantz RP, McNulty SE, Rogers JG, Anderson J, Johnson GW, Walsh MN, Poole JE, Mark DB, Lee KL, Bardy GH. Impact of implantable cardioverter‐defibrillator, amiodarone, and placebo on the mode of death in stable patients with heart failure: analysis from the sudden cardiac death in heart failure trial. Circulation. 2009;120:2170–2176. - PMC - PubMed
Publication types
MeSH terms
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

