PGC-1α in exercise and fasting-induced regulation of hepatic UPR in mice
- PMID: 29961149
- PMCID: PMC6153608
- DOI: 10.1007/s00424-018-2159-3
PGC-1α in exercise and fasting-induced regulation of hepatic UPR in mice
Abstract
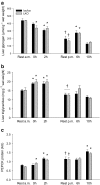
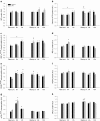
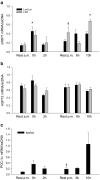
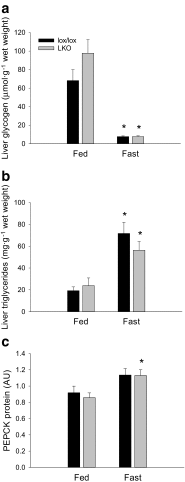
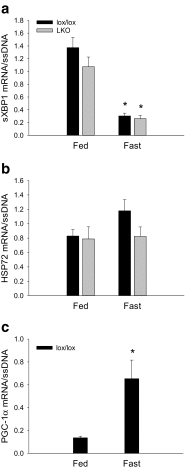
The aim of the present study was to test the hypothesis that PGC-1α is involved in the regulation of hepatic UPR and autophagy in response to both exercise and fasting in mice. Liver-specific PGC-1α knockout (LKO) mice and their floxed littermates (lox/lox) were used in two experimental parts. Liver and plasma were obtained from (1) fed and 18 h fasted mice and (2) immediately after, 2, 6, and 10 h after 1-h treadmill running as well as from resting mice, where one resting group was euthanized at time points corresponding to 0 and 2 h and another corresponding to 6 and 10 h of recovery. Hepatic eIF2α phosphorylation and sXBP1 mRNA content increased immediately after exercise and IRE1α phosphorylation as well as cleaved ATF6 protein content was higher 2 h into recovery than at rest in both genotypes. Fasting reduced hepatic IRE1α phosphorylation and protein content as well as PERK protein and sXBP1 mRNA content similarly in lox/lox and LKO mice. In addition, the hepatic LC3II/LC3I protein ratio increased immediately after exercise and with fasting in both genotypes, while fasting decreased p62 protein content in lox/lox mice. Liver-specific PGC-1α knockout did not affect these responses, but the LC3II/LC3I protein ratio was higher in LKO than lox/lox mice in both rest groups. In conclusion, the present study provides evidence for pathway-specific exercise-induced activation and fasting-induced downregulation of the UPR as well as exercise and fasting-induced regulation of autophagy in mouse liver. In addition, overall PGC-1α does not seem to be required for the fasting and exercise-induced regulation of UPR and autophagy, but may be involved in regulating basal hepatic autophagy.
Keywords: Exercise; Fasting; Liver; PGC-1α; UPR.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



References
-
- Banzet S, Koulmann N, Simler N, Sanchez H, Chapot R, Serrurier B, Peinnequin A, Bigard X. Control of gluconeogenic genes during intense/prolonged exercise: hormone-independent effect of muscle-derived IL-6 on hepatic tissue and PEPCK mRNA. J Appl Physiol. 2009;107:1830–1839. doi: 10.1152/japplphysiol.00739.2009. - DOI - PubMed
-
- Bertholdt L, Gudiksen A, Schwartz CL, Knudsen JG, Pilegaard H. Lack of skeletal muscle IL-6 influences hepatic glucose metabolism in mice during prolonged exercise. Am J Phys Regul Integr Comp Phys. 2017;312:R626–R636. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

