De Novo Missense Variants in TRAF7 Cause Developmental Delay, Congenital Anomalies, and Dysmorphic Features
- PMID: 29961569
- PMCID: PMC6035372
- DOI: 10.1016/j.ajhg.2018.06.005
De Novo Missense Variants in TRAF7 Cause Developmental Delay, Congenital Anomalies, and Dysmorphic Features
Abstract
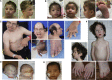
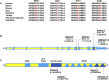
TRAF7 is a multi-functional protein involved in diverse signaling pathways and cellular processes. The phenotypic consequence of germline TRAF7 variants remains unclear. Here we report missense variants in TRAF7 in seven unrelated individuals referred for clinical exome sequencing. The seven individuals share substantial phenotypic overlap, with developmental delay, congenital heart defects, limb and digital anomalies, and dysmorphic features emerging as key unifying features. The identified variants are de novo in six individuals and comprise four distinct missense changes, including a c.1964G>A (p.Arg655Gln) variant that is recurrent in four individuals. These variants affect evolutionarily conserved amino acids and are located in key functional domains. Gene-specific mutation rate analysis showed that the occurrence of the de novo variants in TRAF7 (p = 2.6 × 10-3) and the recurrent de novo c.1964G>A (p.Arg655Gln) variant (p = 1.9 × 10-8) in our exome cohort was unlikely to have occurred by chance. In vitro analyses of the observed TRAF7 mutations showed reduced ERK1/2 phosphorylation. Our findings suggest that missense mutations in TRAF7 are associated with a multisystem disorder and provide evidence of a role for TRAF7 in human development.
Keywords: ERK1/2; MAPKs; RASopathy; TRAF7; cancer; congenital heart defects; de novo missense variants; developmental delay; exome sequencing; limb and digit anomalies.
Copyright © 2018 American Society of Human Genetics. All rights reserved.
Figures

References
-
- Tartaglia M., Mehler E.L., Goldberg R., Zampino G., Brunner H.G., Kremer H., van der Burgt I., Crosby A.H., Ion A., Jeffery S. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat. Genet. 2001;29:465–468. - PubMed
-
- Hanby A.M., Kelsell D.P., Potts H.W., Gillett C.E., Bishop D.T., Spurr N.K., Barnes D.M. Association between loss of heterozygosity of BRCA1 and BRCA2 and morphological attributes of sporadic breast cancer. Int. J. Cancer. 2000;88:204–208. - PubMed
-
- Howlett N.G., Taniguchi T., Olson S., Cox B., Waisfisz Q., De Die-Smulders C., Persky N., Grompe M., Joenje H., Pals G. Biallelic inactivation of BRCA2 in Fanconi anemia. Science. 2002;297:606–609. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

