Structure of HIV TAR in complex with a Lab-Evolved RRM provides insight into duplex RNA recognition and synthesis of a constrained peptide that impairs transcription
- PMID: 29961805
- PMCID: PMC6061845
- DOI: 10.1093/nar/gky529
Structure of HIV TAR in complex with a Lab-Evolved RRM provides insight into duplex RNA recognition and synthesis of a constrained peptide that impairs transcription
Abstract
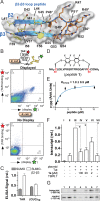
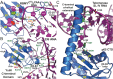
Natural and lab-evolved proteins often recognize their RNA partners with exquisite affinity. Structural analysis of such complexes can offer valuable insight into sequence-selective recognition that can be exploited to alter biological function. Here, we describe the structure of a lab-evolved RNA recognition motif (RRM) bound to the HIV-1 trans-activation response (TAR) RNA element at 1.80 Å-resolution. The complex reveals a trio of arginines in an evolved β2-β3 loop penetrating deeply into the major groove to read conserved guanines while simultaneously forming cation-π and salt-bridge contacts. The observation that the evolved RRM engages TAR within a double-stranded stem is atypical compared to most RRMs. Mutagenesis, thermodynamic analysis and molecular dynamics validate the atypical binding mode and quantify molecular contributions that support the exceptionally tight binding of the TAR-protein complex (KD,App of 2.5 ± 0.1 nM). These findings led to the hypothesis that the β2-β3 loop can function as a standalone TAR-recognition module. Indeed, short constrained peptides comprising the β2-β3 loop still bind TAR (KD,App of 1.8 ± 0.5 μM) and significantly weaken TAR-dependent transcription. Our results provide a detailed understanding of TAR molecular recognition and reveal that a lab-evolved protein can be reduced to a minimal RNA-binding peptide.
Figures




Similar articles
-
Co-crystal structures of HIV TAR RNA bound to lab-evolved proteins show key roles for arginine relevant to the design of cyclic peptide TAR inhibitors.J Biol Chem. 2020 Dec 4;295(49):16470-16486. doi: 10.1074/jbc.RA120.015444. Epub 2020 Oct 13. J Biol Chem. 2020. PMID: 33051202 Free PMC article.
-
The interactions and recognition of cyclic peptide mimetics of Tat with HIV-1 TAR RNA: a molecular dynamics simulation study.J Biomol Struct Dyn. 2013 Mar;31(3):276-87. doi: 10.1080/07391102.2012.698248. Epub 2012 Sep 3. J Biomol Struct Dyn. 2013. PMID: 22943434
-
Face-time with TAR: Portraits of an HIV-1 RNA with diverse modes of effector recognition relevant for drug discovery.J Biol Chem. 2019 Jun 14;294(24):9326-9341. doi: 10.1074/jbc.REV119.006860. Epub 2019 May 12. J Biol Chem. 2019. PMID: 31080171 Free PMC article. Review.
-
An Arg/Lys-rich core peptide mimics TRBP binding to the HIV-1 TAR RNA upper-stem/loop.J Mol Biol. 1998 Jun 26;279(5):1085-99. doi: 10.1006/jmbi.1998.1831. J Mol Biol. 1998. PMID: 9642086
-
Discoveries of Tat-TAR interaction inhibitors for HIV-1.Curr Drug Targets Infect Disord. 2005 Dec;5(4):433-44. doi: 10.2174/156800505774912901. Curr Drug Targets Infect Disord. 2005. PMID: 16535863 Review.
Cited by
-
Co-crystal structures of HIV TAR RNA bound to lab-evolved proteins show key roles for arginine relevant to the design of cyclic peptide TAR inhibitors.J Biol Chem. 2020 Dec 4;295(49):16470-16486. doi: 10.1074/jbc.RA120.015444. Epub 2020 Oct 13. J Biol Chem. 2020. PMID: 33051202 Free PMC article.
-
Full-Length NAD+-I Riboswitches Bind a Single Cofactor but Cannot Discriminate against Adenosine Triphosphate.Biochemistry. 2023 Dec 5;62(23):3396-3410. doi: 10.1021/acs.biochem.3c00391. Epub 2023 Nov 10. Biochemistry. 2023. PMID: 37947391 Free PMC article.
-
Constrained peptides mimic a viral suppressor of RNA silencing.Nucleic Acids Res. 2021 Dec 16;49(22):12622-12633. doi: 10.1093/nar/gkab1149. Nucleic Acids Res. 2021. PMID: 34871435 Free PMC article.
-
Structural and computational studies of HIV-1 RNA.RNA Biol. 2024 Jan;21(1):1-32. doi: 10.1080/15476286.2023.2289709. Epub 2023 Dec 15. RNA Biol. 2024. PMID: 38100535 Free PMC article. Review.
-
Advances in chaperone-assisted RNA crystallography using synthetic antibodies.BBA Adv. 2023 Aug 19;4:100101. doi: 10.1016/j.bbadva.2023.100101. eCollection 2023. BBA Adv. 2023. PMID: 37655005 Free PMC article.
References
-
- Fauci A., Dieffenbach C.. Thirty Years of HIV and AIDS: future challenges and opportunities. Ann. Intern. Med. 2011; 154:766–771. - PubMed
-
- HIV/AIDS, J.U.N.P.o. 2016; Geneva: www.unaids.org/sites/default/files/media_asset/global-AIDS-update-2016_e....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

