Community-based Malaria Screening and Treatment for Pregnant Women Receiving Standard Intermittent Preventive Treatment With Sulfadoxine-Pyrimethamine: A Multicenter (The Gambia, Burkina Faso, and Benin) Cluster-randomized Controlled Trial
- PMID: 29961848
- PMCID: PMC6355825
- DOI: 10.1093/cid/ciy522
Community-based Malaria Screening and Treatment for Pregnant Women Receiving Standard Intermittent Preventive Treatment With Sulfadoxine-Pyrimethamine: A Multicenter (The Gambia, Burkina Faso, and Benin) Cluster-randomized Controlled Trial
Abstract
Background: We investigated whether adding community scheduled malaria screening and treatment (CSST) with artemether-lumefantrine by community health workers (CHWs) to standard intermittent preventive treatment in pregnancy with sulfadoxine-pyrimethamine (IPTp-SP) would improve maternal and infant health.
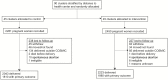
Methods: In this 2-arm cluster-randomized, controlled trial, villages in Burkina Faso, The Gambia, and Benin were randomized to receive CSST plus IPTp-SP or IPTp-SP alone. CHWs in the intervention arm performed monthly CSST during pregnancy. At each contact, filter paper and blood slides were collected, and at delivery, a placental biopsy was collected. Primary and secondary endpoints were the prevalence of placental malaria, maternal anemia, maternal peripheral infection, low birth weight, antenatal clinic (ANC) attendance, and IPTp-SP coverage.
Results: Malaria infection was detected at least once for 3.8% women in The Gambia, 16.9% in Benin, and 31.6% in Burkina Faso. There was no difference between study arms in terms of placenta malaria after adjusting for birth season, parity, and IPTp-SP doses (adjusted odds ratio, 1.06 [95% confidence interval, .78-1.44]; P = .72). No difference between the study arms was found for peripheral maternal infection, anemia, and adverse pregnancy outcomes. ANC attendance was significantly higher in the intervention arm in Burkina Faso but not in The Gambia and Benin. Increasing number of IPTp-SP doses was associated with a significantly lower risk of placenta malaria, anemia at delivery, and low birth weight.
Conclusions: Adding CSST to existing IPTp-SP strategies did not reduce malaria in pregnancy. Increasing the number of IPTp-SP doses given during pregnancy is a priority.
Clinical trials registration: NCT01941264; ISRCTN37259296.
Figures

References
-
- Rogerson SJ, Desai M, Mayor A, Sicuri E, Taylor SM, van Eijk AM. Burden, pathology, and costs of malaria in pregnancy: new developments for an old problem. Lancet Infect Dis 2018; 18:e107–18. - PubMed
-
- Hartman TK, Rogerson SJ, Fischer PR. The impact of maternal malaria on newborns. Ann Trop Paediatr 2010; 30:271–82. - PubMed
-
- World Health Organization. Guidelines for the treatment of malaria. 3rd ed. Geneva, Switzerland: WHO, 2015.
-
- van Eijk AM, Hill J, Larsen DA, et al. . Coverage of intermittent preventive treatment and insecticide-treated nets for the control of malaria during pregnancy in sub-Saharan Africa: a synthesis and meta-analysis of national survey data, 2009–11. Lancet Infect Dis 2013; 13:1029–42. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

