Comparison of Ketamine with Midazolam versus Ketamine with Fentanyl for Pediatric Extracorporeal Shock Wave Lithotripsy Procedure: A Randomized Controlled Study
- PMID: 29962617
- PMCID: PMC6020612
- DOI: 10.4103/aer.AER_44_18
Comparison of Ketamine with Midazolam versus Ketamine with Fentanyl for Pediatric Extracorporeal Shock Wave Lithotripsy Procedure: A Randomized Controlled Study
Abstract
Objectives: To compare the effects of ketamine-fentanyl (KF) and ketamine-midazolam (KM) combinations on hemodynamic parameters, recovery properties, pain, and side effects in pediatric patients undergoing extracorporeal shock wave lithotripsy (ESWL) procedure.
Methodology: In this double-blinded, randomized trial, 60 pediatric patients aged between 1 and 13 years with American Society of Anesthesiologists physical status Classes I and II, who scheduled for ESWL procedure, were included in the study. Patients were randomly divided into two groups: Group KM received 0.1 mg/kg of midazolam +1-1.5 mg/kg of ketamine and Group KF received 1 μg/kg of fentanyl +1-1.5 mg/kg of ketamine intravenously.
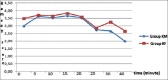
Results: There were similar demographic properties, recovery, and discharge times between groups. No statistically significant difference was found in peripheral oxygen saturation, mean and diastolic blood pressure, Ramsey sedation scores, modified Aldrete recovery scores, side effects, and recovery times (Group KM, 16.067 ± 1.2 min; Group KF, 19.46 ± 0.86 min) between groups (P > 0.05).
Conclusion: KF combination offers better hemodynamic properties, less side effects with lower visual analog scores, and face, legs, activity, cry, and consolability scores than KM in the pediatric ESWL procedure.
Keywords: Child; fentanyl; ketamine; lithotripsy; midazolam; pain.
Conflict of interest statement
There are no conflicts of interest.
Figures


Comment in
-
Re: Comparison of Ketamine with Midazolam versus Ketamine with Fentanyl for Pediatric Extracorporeal Shock Wave Lithotripsy Procedure: A Randomized Controlled Study.J Urol. 2019 Jan;201(1):26. doi: 10.1097/01.ju.0000550149.39487.03. J Urol. 2019. PMID: 30577369 No abstract available.
Similar articles
-
Dexmedetomidine-ketamine and midazolam-ketamine combinations for sedation in pediatric patients undergoing extracorporeal shock wave lithotripsy: a randomized prospective study.J Anesth. 2010 Dec;24(6):858-63. doi: 10.1007/s00540-010-1023-1. Epub 2010 Oct 6. J Anesth. 2010. PMID: 20924617 Clinical Trial.
-
Effects of intravenous ketorolac and fentanyl combined with midazolam on analgesia and side effects during extracorporeal shock wave lithotripsy.Acta Anaesthesiol Sin. 2002 Mar;40(1):9-12. Acta Anaesthesiol Sin. 2002. PMID: 11989050 Clinical Trial.
-
Addition of low-dose ketamine to midazolam-fentanyl-propofol-based sedation for colonoscopy: a randomized, double-blind, controlled trial.J Clin Anesth. 2015 Jun;27(4):301-6. doi: 10.1016/j.jclinane.2015.03.017. Epub 2015 Mar 20. J Clin Anesth. 2015. PMID: 25801162 Clinical Trial.
-
Comparison of the Effectiveness of Dexmedetomidine-Ketamine and Midazolam-Ketamine Regimens in Sedation of Children Treated with Extracorporeal Shock Wave Lithotripsy.Anesth Pain Med. 2023 Apr 13;13(3):e129776. doi: 10.5812/aapm-129776. eCollection 2023 Jun. Anesth Pain Med. 2023. PMID: 38021338 Free PMC article.
-
Assessment of the effects of ketamine-fentanyl combination versus propofol-remifentanil combination for sedation during endoscopic retrograde cholangiopancreatography.J Res Med Sci. 2014 Sep;19(9):860-6. J Res Med Sci. 2014. PMID: 25535501 Free PMC article.
Cited by
-
Comparison of extracorporeal shockwave lithotripsy and retrograde intrarenal surgery in the treatment of renal pelvic and proximal ureteral stones ≤2 cm in children.Indian J Urol. 2020 Oct-Dec;36(4):282-287. doi: 10.4103/iju.IJU_116_20. Epub 2020 Oct 1. Indian J Urol. 2020. PMID: 33376264 Free PMC article.
-
Safety and Efficacy of Ketamine Versus Ketamine-Fentanyl-Dexmedetomidine Combination for Anesthesia and Analgesia in Rats.Dose Response. 2019 Feb 12;17(1):1559325819825902. doi: 10.1177/1559325819825902. eCollection 2019 Jan-Mar. Dose Response. 2019. PMID: 30792614 Free PMC article.
References
-
- Vandervoort K, Wiesen J, Frank R, Vento S, Crosby V, Chandra M, et al. Urolithiasis in pediatric patients: A single center study of incidence, clinical presentation and outcome. J Urol. 2007;177:2300–5. - PubMed
-
- Onal B, Citgez S, Tansu N, Emin G, Demirkesen O, Talat Z, et al. What changed in the management of pediatric stones after the introduction of minimally invasive procedures? A single-center experience over 24 years. J Pediatr Urol. 2013;9:910–4. - PubMed
-
- Koruk S, Mizrak A, Gul R, Kilic E, Yendi F, Oner U, et al. Dexmedetomidine-ketamine and midazolam-ketamine combinations for sedation in pediatric patients undergoing extracorporeal shock wave lithotripsy: A randomized prospective study. J Anesth. 2010;24:858–63. - PubMed
-
- Torrecilla Ortiz C, Rodríguez Blanco LL, Díaz Vicente F, González Satué C, Marco Pérez LM, Trilla Herrera E, et al. Extracorporeal shock-wave lithotripsy: Anxiety and pain perception. Actas Urol Esp. 2000;24:163–8. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

