Tumor heterogeneity of gastric cancer: From the perspective of tumor-initiating cell
- PMID: 29962814
- PMCID: PMC6021770
- DOI: 10.3748/wjg.v24.i24.2567
Tumor heterogeneity of gastric cancer: From the perspective of tumor-initiating cell
Abstract
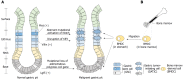
Gastric cancer (GC) remains one of the most common and malignant types of cancer due to its rapid progression, distant metastasis, and resistance to conventional chemotherapy, although efforts have been made to understand the underlying mechanism of this resistance and to improve clinical outcome. It is well recognized that tumor heterogeneity, a fundamental feature of malignancy, plays an essential role in the cancer development and chemoresistance. The model of tumor-initiating cell (TIC) has been proposed to explain the genetic, histological, and phenotypical heterogeneity of GC. TIC accounts for a minor subpopulation of tumor cells with key characteristics including high tumorigenicity, maintenance of self-renewal potential, giving rise to both tumorigenic and non-tumorigenic cancer cells, and resistance to chemotherapy. Regarding tumor-initiating cell of GC (GATIC), substantial studies have been performed to (1) identify the putative specific cell markers for purification and functional validation of GATICs; (2) trace the origin of GATICs; and (3) decode the regulatory mechanism of GATICs. Furthermore, recent studies demonstrate the plasticity of GATIC and the interaction between GATIC and its surrounding factors (TIC niche or tumor microenvironment). All these investigations pave the way for the development of GATIC-targeted therapy, which is in the phase of preclinical studies and clinical trials. Here, we interpret the heterogeneity of GC from the perspectives of TIC by reviewing the above-mentioned fundamental and clinical studies of GATICs. Problems encountered during the GATIC investigations and the potential solutions are also discussed.
Keywords: Gastric cancer; Tumor heterogeneity; Tumor-initiating cell.
Conflict of interest statement
Conflict-of-interest statement: No potential conflicts of interest.
Figures

References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. - PubMed
-
- Saikawa Y, Fukuda K, Takahashi T, Nakamura R, Takeuchi H, Kitagawa Y. Gastric carcinogenesis and the cancer stem cell hypothesis. Gastric Cancer. 2010;13:11–24. - PubMed
-
- Marin JJ, Al-Abdulla R, Lozano E, Briz O, Bujanda L, Banales JM, Macias RI. Mechanisms of Resistance to Chemotherapy in Gastric Cancer. Anticancer Agents Med Chem. 2016;16:318–334. - PubMed
-
- Thuss-Patience PC, Kretzschmar A, Bichev D, Deist T, Hinke A, Breithaupt K, Dogan Y, Gebauer B, Schumacher G, Reichardt P. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer--a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO) Eur J Cancer. 2011;47:2306–2314. - PubMed
-
- Kang JH, Lee SI, Lim DH, Park KW, Oh SY, Kwon HC, Hwang IG, Lee SC, Nam E, Shin DB, et al. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol. 2012;30:1513–1518. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

