Chromatin Remodeling Proteins in Epilepsy: Lessons From CHD2-Associated Epilepsy
- PMID: 29962935
- PMCID: PMC6013553
- DOI: 10.3389/fnmol.2018.00208
Chromatin Remodeling Proteins in Epilepsy: Lessons From CHD2-Associated Epilepsy
Abstract
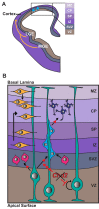
The chromodomain helicase DNA-binding (CHD) family of proteins are ATP-dependent chromatin remodelers that contribute to the reorganization of chromatin structure and deposition of histone variants necessary to regulate gene expression. CHD proteins play an important role in neurodevelopment, as pathogenic variants in CHD1, CHD2, CHD4, CHD7 and CHD8 have been associated with a range of neurological phenotypes, including autism spectrum disorder (ASD), intellectual disability (ID) and epilepsy. Pathogenic variants in CHD2 are associated with developmental epileptic encephalopathy (DEE) in humans, however little is known about how these variants contribute to this disorder. Of the nine CHD family members, CHD2 is the only one that leads to a brain-restricted phenotype when disrupted in humans. This suggests that despite being expressed ubiquitously, CHD2 has a unique role in human brain development and function. In this review, we will discuss the phenotypic spectrum of patients with pathogenic variants in CHD2, current animal models of CHD2 deficiency, and the role of CHD2 in proliferation, neurogenesis, neuronal differentiation, chromatin remodeling and DNA-repair. We also consider how CHD2 depletion can affect each of these biological mechanisms and how these defects may underpin neurodevelopmental disorders including epilepsy.
Keywords: CHD2; chromatin remodeler; epigenetics; epilepsy genetics; neurodevelopment.
Figures


References
-
- Anderson S. A., Marin O., Horn C., Jennings K., Rubenstein J. L. (2001). Distinct cortical migrations from the medial and lateral ganglionic eminences. Development 128, 353–363. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

