Nab-paclitaxel is effective against intrahepatic cholangiocarcinoma via disruption of desmoplastic stroma
- PMID: 29963132
- PMCID: PMC6019977
- DOI: 10.3892/ol.2018.8690
Nab-paclitaxel is effective against intrahepatic cholangiocarcinoma via disruption of desmoplastic stroma
Abstract
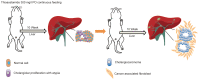
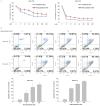
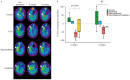
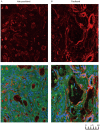
Intrahepatic cholangiocarcinoma (IH-CCA) is the second predominant hepatic malignancy worldwide. However, effective treatment strategies for IH-CCA have not yet been developed. Nab-paclitaxel may be an effective drug against IH-CCA, a type of desmoid-like tumor, and its antitumor effects may be attributable to its ability to disrupt the cancer-associated fibroblasts. In the present study, MTT and Annexin-V apoptosis detection kits were used to evaluate the efficacy of paclitaxel and nab-paclitaxel against human cholangiocarcinoma KKU-100 and KKU-213 cell lines. A rat model of thioacetamide-induced spontaneous desmoplastic IH-CCA was used to compare the treatment response of four different drug regimens: Control, paclitaxel, nab-paclitaxel and gemcitabine/oxaliplatin. Positron emission tomography and immunofluorescence analysis were used to measure the tumor volume and to study the resected tumor, respectively. In vitro, paclitaxel and nab-paclitaxel induced anti-proliferative effects in KKU-100 and KKU-M213 cells. With regards to the treatment regimes, only nab-paclitaxel and gemcitabine/oxaliplatin induced antitumor effects in the rat model of thioacetamide-induced IH-CCA. The immunofluorescence study indicated that nab-paclitaxel was more efficient in disrupting cancer-associated fibroblasts than paclitaxel. In conclusion, nab-paclitaxel is effective against IH-CCA owing to its ability to markedly disrupt the desmoplastic stroma.
Keywords: cancer-associated fibroblast; cholangiocarcinoma; desmoplastic; nab-paclitaxel; α-smooth muscle actin.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
