Chemical synthesis, characterisation and in vitro and in vivo metabolism of the synthetic opioid MT-45 and its newly identified fluorinated analogue 2F-MT-45 with metabolite confirmation in urine samples from known drug users
- PMID: 29963206
- PMCID: PMC6002428
- DOI: 10.1007/s11419-018-0413-1
Chemical synthesis, characterisation and in vitro and in vivo metabolism of the synthetic opioid MT-45 and its newly identified fluorinated analogue 2F-MT-45 with metabolite confirmation in urine samples from known drug users
Abstract
Purpose: The detection of a novel psychoactive substance, 2F-MT-45, a fluorinated analogue of the synthetic opioid MT-45, was reported in a single seized tablet. MT-45, 2F-, 3F- and 4F-MT-45 were synthesised and reference analytical data were reported. The in vitro and in vivo metabolisms of MT-45 and 2F-MT-45 were investigated.
Method: The reference standards and seized sample were characterised using nuclear magnetic resonance spectroscopy, ultra-performance liquid chromatography-quadrupole time of flight mass spectrometry, gas chromatography-mass spectrometry, attenuated total reflectance-Fourier transform infrared spectroscopy and Raman spectroscopy. Presumptive tests were performed and physicochemical properties of the compounds determined. Metabolite identification studies using human liver microsomes, human hepatocytes, mouse hepatocytes and in vivo testing using mice were performed and identified MT-45 metabolites were confirmed in authentic human urine samples.
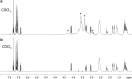
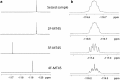
Results: Metabolic pathways identified for MT-45 and 2F-MT-45 were N-dealkylation, hydroxylation and subsequent glucuronidation. The major MT-45 metabolites identified in human in vitro studies and in authenticated human urine were phase I metabolites and should be incorporated as analytical targets to existing toxicological screening methods. Phase II glucuronidated metabolites were present in much lower proportions.
Conclusions: 2F-MT-45 has been detected in a seized tablet for the first time. The metabolite identification data provide useful urinary metabolite targets for forensic and clinical testing for MT-45 and allows screening of urine for 2F-MT-45 and its major metabolites to determine its prevalence in case work.
Keywords: 2F-MT-45; Clinical and forensic toxicology; MT-45; Metabolite identification; Novel psychoactive substances; Synthetic opioids.
Conflict of interest statement
Compliance with ethical standardsThere are no financial or other relations that could lead to a conflict of interest.All procedures performed in this study involving human participants were in accordance with the ethical standards of the international and/or national committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The two authentic human urine samples were collected as part of the STRIDA project which is conducted in accordance with the Helsinki Declaration and was approved by the regional ethical review board (Nr. 2013/116–31/2). All regulated procedures on living animals at the University of Dundee were carried out under the authority of a project licence issued by the Home Office under the Animals (Scientific Procedures) Act 1986, as amended in 2012 (and in compliance with EU Directive EU/2010/63). Licence applications were approved by the University’s Ethical Review Committee (ERC) before submission to the Home Office. The ERC has a general remit to develop and oversee policy on all aspects of the use of animals on University premises and is a subcommittee of the University Court, its highest governing body.
Figures





References
-
- EMCDDA (2014) EMCDDA–Europol Joint Report on a new psychoactive substance: 1-cyclohexyl-4-(1,2-diphenylethyl)piperazine (‘MT-45’). http://www.emcdda.europa.eu/system/files/publications/810/TDAS14007ENN_4.... Accessed 25 Aug 2017
-
- European Patent Office (1976) 1-Substituted-4-(1,2-diphenylethyl)piperazine derivatives and their salts and the preparation thereof. US Patent 3,957,788. Issued 18th May 1976. https://worldwide.espacenet.com/publicationDetails/biblio?CC=US&NR=39577.... Accessed 21 Aug 2017
-
- Nakamura H, Shimizu M. Comparative study of 1-(cyclohexyl-4-(1,2-diphenylethyl)- piperazine (MT-45) and its enantiomorphs on analgesic and other pharmacological activities in experimental animals. Arch Int Pharmacodyn Ther. 1976;221:105–121. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

