Cancer stem cells from peritumoral tissue of glioblastoma multiforme: the possible missing link between tumor development and progression
- PMID: 29963265
- PMCID: PMC6021333
- DOI: 10.18632/oncotarget.25565
Cancer stem cells from peritumoral tissue of glioblastoma multiforme: the possible missing link between tumor development and progression
Abstract
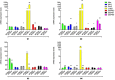
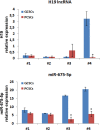
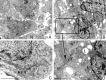
In glioblastoma multiforme (GBM), cancer stem cells (CSCs) are thought to be responsible for gliomagenesis, resistance to treatment and recurrence. Unfortunately, the prognosis for GBM remains poor and recurrence frequently occurs in the peritumoral tissue within 2 cm from the tumor edge. In this area, a population of CSCs has been demonstrated which may recapitulate the tumor after surgical resection. In the present study, we aimed to characterize CSCs derived from both peritumoral tissue (PCSCs) and GBM (GCSCs) in order to deepen their significance in GBM development and progression. The stemness of PCSC/GCSC pairs obtained from four human GBM surgical specimens was investigated by comparing the expression of specific stem cell markers such as Nestin, Musashi-1 and SOX2. In addition, the growth rate, the ultrastructural features and the expression of other molecules such as c-Met, pMet and MAP kinases, involved in cell migration/invasion, maintenance of tumor stemness and/or resistance to treatments were evaluated. Since it has been recently demonstrated the involvement of the long non-coding RNAs (lncRNAs) in the progression of gliomas, the expression of H19 lncRNA, as well as of one of its two mature products miR-675-5p was evaluated in neurospheres. Our results show significant differences between GCSCs and PCSCs in terms of proliferation, ultrastructural peculiarities and, at a lower extent, stemness profile. These differences might be important in view of their potential role as a therapeutic target.
Keywords: H19 lncRNA and miR-675-5p; glioblastoma cancer stem cells; peritumoral cancer stem cells; proliferation and invasiveness markers; stemness markers.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflicts of interest.
Figures



References
-
- Denysenko T, Gennero L, Roos MA, Melcarne A, Juenemann C, Faccani G, Morra I, Cavallo G, Reguzzi S, Pescarmona G, Ponzetto A. Glioblastoma cancer stem cells: heterogeneity, microenvironment and related therapeutic strategies. Cell Biochem Funct. 2010;28:343–351. - PubMed
-
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. - PubMed
-
- Eramo A, Ricci-Vitiani L, Zeuner A, Pallini R, Lotti F, Sette G, Pilozzi E, Larocca LM, Peschle C, De Maria R. Chemotherapy resistance of glioblastoma stem cells. Cell Death Differ. 2006;13:1238–1241. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

