Advanced Plant-Based Glycan Engineering
- PMID: 29963553
- PMCID: PMC6010556
- DOI: 10.3389/fbioe.2018.00081
Advanced Plant-Based Glycan Engineering
Abstract
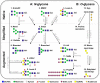
With respect to biomanufacturing, glycosylation is one of the most addressed post-translational modifications, since it is well-known that the attachment of sugar residues efficiently affects protein homogeneity and functionality. Much effort has been taken into engineering various expression systems to control glycosylation and to generate molecules with targeted sugar profiles. Nevertheless, engineering of N- and O-linked glycans on well-established expression systems remains challenging. On the one side the glycosylation machinery in mammalian cells is hard to control due to its complexity. Most bacteria, on the other side, completely lack such glycan formations, and in general exhibit fundamental differences in their glycosylation abilities. Beyond that, plants generate complex N-glycans typical of higher eukaryotes, but simpler than those produced by mammals. Paradoxically, it seems that the limited glycosylation capacity of plant cells is an advantage for specific glycan manipulations. This review focuses on recent achievements in plant glycan engineering and provides a short outlook on how new developments (in synthetic biology) might have a positive impact.
Keywords: N- and O-linked glycans; glycan engineering; glyco proteins; glycosylation; plant-biotechnology; plants.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

