Permselectivity limits of biomimetic desalination membranes
- PMID: 29963628
- PMCID: PMC6025908
- DOI: 10.1126/sciadv.aar8266
Permselectivity limits of biomimetic desalination membranes
Abstract
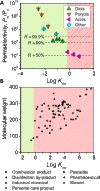
Water scarcity and inadequate membrane selectivity have spurred interest in biomimetic desalination membranes, in which biological or synthetic water channels are incorporated in an amphiphilic bilayer. As low channel densities (0.1 to 10%) are required for sufficient water permeability, the amphiphilic bilayer matrix will play a critical role in separation performance. We determine selectivity limits for biomimetic membranes by studying the transport behavior of water, neutral solutes, and ions through the bilayers of lipid and block-copolymer vesicles and projecting performance for varying water channel densities. We report that defect-free biomimetic membranes would have water/salt permselectivities ~108-fold greater than current desalination membranes. In contrast, the solubility-based permeability of lipid and block-copolymer bilayers (extending Overton's rule) will result in poor rejection of hydrophobic solutes. Defect-free biomimetic membranes thus offer great potential for seawater desalination and ultrapure water production, but would perform poorly in wastewater reuse. Potential strategies to limit neutral solute permeation are discussed.
Figures



References
-
- Elimelech M., Phillip W. A., The future of seawater desalination: Energy, technology, and the environment. Science 333, 712–717 (2011). - PubMed
-
- Werber J. R., Osuji C. O., Elimelech M., Materials for next-generation desalination and water purification membranes. Nat. Rev. Mater. 1, 16018 (2016).
-
- National Research Council, Water Reuse: Potential for Expanding the Nation’s Water Supply Through Reuse of Municipal Wastewater (National Academies Press, 2012).
-
- Werber J. R., Deshmukh A., Elimelech M., The critical need for increased selectivity, not increased water permeability, for desalination membranes. Environ. Sci. Technol. Lett. 3, 112–120 (2016).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

