Macrophage targeting: opening new possibilities for cancer immunotherapy
- PMID: 29963704
- PMCID: PMC6187207
- DOI: 10.1111/imm.12976
Macrophage targeting: opening new possibilities for cancer immunotherapy
Abstract
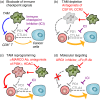
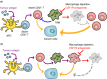
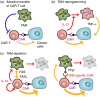
Tumour-infiltrating immune cells regulate tumour development and progression either negatively or positively. For example, cytotoxic lymphocytes (CTL) such as CD8+ T and natural killer (NK) cells can recognize and eliminate cancer cells, and thereby restrict the tumour growth and metastasis, if they exert full cytotoxicity. In contrast, tumour-infiltrating myeloid cells such as tumour-associated macrophages (TAM) promote the expansion and dissemination of cancer cells depending on their functional states. Given the tumour-killing ability of CTL, the augmentation of CTL-induced antitumour immune reactions has been considered as an attractive therapeutic modality for lethal solid tumours and several promising strategies have emerged, which include immune checkpoint inhibitors, cancer vaccines and adoptive CTL transfer. These immunotherapies are now tested in clinical trials and have shown significant antitumour effects in patients with lymphoma and some solid tumours such as melanoma and lung cancer. Despite these encouraging results, these therapies are not efficient in a certain fraction of patients and tumour types with tumour cell-intrinsic mechanisms such as impaired antigen presentation and/or tumour cell-extrinsic mechanisms including the accumulation of immunosuppressive cells. Several animal studies suggest that tumour-infiltrating myeloid cells, especially TAM, are one of the key targets to improve the efficacy of immunotherapies as these cells can suppress the functions of CD8+ T and NK cells. In this review, we will summarize recent animal studies regarding the involvement of TAM in the immune checkpoint, cancer vaccination and adoptive CTL transfer therapies, and discuss the therapeutic potential of TAM targeting to improve the immunotherapies.
Keywords: cancer; immunotherapy; macrophage; tumour immunology.
© 2018 The Authors. Immunology Published by John Wiley & Sons Ltd.
Figures

References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017; 67:7–30. - PubMed
-
- Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004; 21:137–48. - PubMed
-
- Ljunggren HG, Malmberg KJ. Prospects for the use of NK cells in immunotherapy of human cancer. Nat Rev Immunol 2007; 7:329–39. - PubMed
-
- Kershaw MH, Westwood JA, Darcy PK. Gene‐engineered T cells for cancer therapy. Nat Rev Cancer 2013; 13:525–41. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

