FOXO are required for intervertebral disk homeostasis during aging and their deficiency promotes disk degeneration
- PMID: 29963746
- PMCID: PMC6156454
- DOI: 10.1111/acel.12800
FOXO are required for intervertebral disk homeostasis during aging and their deficiency promotes disk degeneration
Abstract
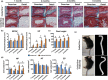
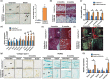
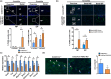
Intervertebral disk (IVD) degeneration is a prevalent age-associated musculoskeletal disorder and a major cause of chronic low back pain. Aging is the main risk factor for the disease, but the molecular mechanisms regulating IVD homeostasis during aging are unknown. The aim of this study was to investigate the function of FOXO, a family of transcription factors linked to aging and longevity, in IVD aging and age-related degeneration. Conditional deletion of all FOXO isoforms (FOXO1, 3, and 4) in IVD using the Col2a1Cre and AcanCreER mouse resulted in spontaneous development of IVD degeneration that was driven by severe cell loss in the nucleus pulposus (NP) and cartilaginous endplates (EP). Conditional deletion of individual FOXO in mature mice showed that FOXO1 and FOXO3 are the dominant isoforms and have redundant functions in promoting IVD homeostasis. Gene expression analyses indicated impaired autophagy and reduced antioxidant defenses in the NP of FOXO-deficient IVD. In primary human NP cells, FOXO directly regulated autophagy and adaptation to hypoxia and promoted resistance to oxidative and inflammatory stress. Our findings demonstrate that FOXO are critical regulators of IVD homeostasis during aging and suggest that maintaining or restoring FOXO expression can be a therapeutic strategy to promote healthy IVD aging and delay the onset of IVD degeneration.
Keywords: FOXO; aging; autophagy; intervertebral disk.
© 2018 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Figures



References
-
- Alvarez‐Garcia, O. , Matsuzaki, T. , Olmer, M. , Plate, L. , Kelly, J. W. , & Lotz, M. K. (2017). Regulated in development and DNA damage response 1 deficiency impairs autophagy and mitochondrial biogenesis in articular cartilage and increases the severity of experimental osteoarthritis. Arthritis & Rheumatology, 69, 1418–1428. - PMC - PubMed
-
- Ambrogini, E. , Almeida, M. , Martin‐Millan, M. , Paik, J. H. , Depinho, R. A. , Han, L. , … Manolagas, S. C. (2010). FoxO‐mediated defense against oxidative stress in osteoblasts is indispensable for skeletal homeostasis in mice. Cell Metabolism, 11, 136–146. 10.1016/j.cmet.2009.12.009 - DOI - PMC - PubMed
-
- Anderson, D. G. , & Tannoury, C. (2005). Molecular pathogenic factors in symptomatic disc degeneration. The Spine Journal, 5, 260S–266S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

