The combination of ribose and adenine promotes adenosine release and attenuates the intensity and frequency of epileptiform activity in hippocampal slices: Evidence for the rapid depletion of cellular ATP during electrographic seizures
- PMID: 29964329
- PMCID: PMC6220757
- DOI: 10.1111/jnc.14543
The combination of ribose and adenine promotes adenosine release and attenuates the intensity and frequency of epileptiform activity in hippocampal slices: Evidence for the rapid depletion of cellular ATP during electrographic seizures
Abstract
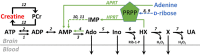
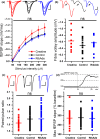
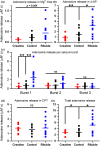
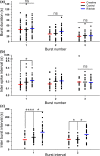
In addition to being the universal cellular energy source, ATP is the primary reservoir for the neuromodulator adenosine. Consequently, adenosine is produced during ATP-depleting conditions, such as epileptic seizures, during which adenosine acts as an anticonvulsant to terminate seizure activity and raise the threshold for subsequent seizures. These actions protect neurones from excessive ionic fluxes and hence preserve the remaining cellular content of ATP. We have investigated the consequences of manipulation of intracellular ATP levels on adenosine release and epileptiform activity in hippocampal slices by pre-incubating slices (3 h) with creatine (1 mM) and the combination of ribose (1 mM) and adenine (50 μM; RibAde). Creatine buffers and protects the concentration of cellular ATP, whereas RibAde restores the reduced cellular ATP in brain slices to near physiological levels. Using electrophysiological recordings and microelectrode biosensors for adenosine, we find that, while having no effect on basal synaptic transmission or paired-pulse facilitation, pre-incubation with creatine reduced adenosine release during Mg2+- free/4-aminopyridine-induced electrographic seizure activity, whereas RibAde increased adenosine release. This increased release of adenosine was associated with an attenuation of both the intensity and frequency of seizure activity. Given the depletion of ATP after injury to the brain, the propensity for seizures after trauma and the risk of epileptogenesis, therapeutic strategies elevating the cellular reservoir of adenosine may have value in the traumatized brain. Ribose and adenine are both in use in man and thus their combination merits consideration as a potential therapeutic for the acutely injured central nervous system.
Keywords: ATP; Adenosine; RibAde; epilepsy; purines; seizures.
© The Authors. Journal of Neurochemistry published by John Wiley & Sons Ltd on behalf of International Society for Neurochemistry.
Figures

Similar articles
-
Modulation of intracellular ATP determines adenosine release and functional outcome in response to metabolic stress in rat hippocampal slices and cerebellar granule cells.J Neurochem. 2014 Jan;128(1):111-24. doi: 10.1111/jnc.12397. Epub 2013 Sep 12. J Neurochem. 2014. PMID: 23937448
-
The Purine Salvage Pathway and the Restoration of Cerebral ATP: Implications for Brain Slice Physiology and Brain Injury.Neurochem Res. 2019 Mar;44(3):661-675. doi: 10.1007/s11064-017-2386-6. Epub 2017 Aug 24. Neurochem Res. 2019. PMID: 28836168 Free PMC article. Review.
-
Intracellular ATP influences synaptic plasticity in area CA1 of rat hippocampus via metabolism to adenosine and activity-dependent activation of adenosine A1 receptors.J Neurosci. 2011 Apr 20;31(16):6221-34. doi: 10.1523/JNEUROSCI.4039-10.2011. J Neurosci. 2011. PMID: 21508245 Free PMC article.
-
Adenine/ribose supply increases adenosine production and protects ATP pool in adenosine kinase-inhibited cardiac cells.J Mol Cell Cardiol. 1998 Mar;30(3):673-83. doi: 10.1006/jmcc.1997.0635. J Mol Cell Cardiol. 1998. PMID: 9515042
-
ATP release during seizures - A critical evaluation of the evidence.Brain Res Bull. 2019 Sep;151:65-73. doi: 10.1016/j.brainresbull.2018.12.021. Epub 2019 Jan 17. Brain Res Bull. 2019. PMID: 30660718 Review.
Cited by
-
Neuronal Loss of the Glutamate Transporter GLT-1 Promotes Excitotoxic Injury in the Hippocampus.Front Cell Neurosci. 2021 Dec 29;15:788262. doi: 10.3389/fncel.2021.788262. eCollection 2021. Front Cell Neurosci. 2021. PMID: 35035352 Free PMC article.
-
The protective role of commensal gut microbes and their metabolites against bacterial pathogens.Gut Microbes. 2024 Jan-Dec;16(1):2356275. doi: 10.1080/19490976.2024.2356275. Epub 2024 May 26. Gut Microbes. 2024. PMID: 38797999 Free PMC article.
-
Adenosine and P1 receptors: Key targets in the regulation of sleep, torpor, and hibernation.Front Pharmacol. 2023 Mar 10;14:1098976. doi: 10.3389/fphar.2023.1098976. eCollection 2023. Front Pharmacol. 2023. PMID: 36969831 Free PMC article. Review.
-
Research progress on adenosine in central nervous system diseases.CNS Neurosci Ther. 2019 Sep;25(9):899-910. doi: 10.1111/cns.13190. Epub 2019 Jul 23. CNS Neurosci Ther. 2019. PMID: 31334608 Free PMC article. Review.
-
Adenosinergic Pathway in Parkinson's Disease: Recent Advances and Therapeutic Perspective.Mol Neurobiol. 2023 Jun;60(6):3054-3070. doi: 10.1007/s12035-023-03257-3. Epub 2023 Feb 14. Mol Neurobiol. 2023. PMID: 36786912 Review.
References
-
- Anderson W. W. and Collingridge G. L. (2007) Capabilities of the WinLTP data acquisition program extending beyond basic LTP experimental functions. J. Neurosci. Methods 162, 346–356. - PubMed
-
- Andres R. H., Ducray A. D., Schlattner U., Wallimann T. and Widmer H. R. (2008) Functions and effects of creatine in the central nervous system. Brain Res. Bull. 76, 329–343. - PubMed
-
- Avsar E. and Empson R. M. (2004) Adenosine acting via A1 receptors, controls the transition to status epilepticus‐like behaviour in an in vitro model of epilepsy. Neuropharmacology 47, 427–437. - PubMed
-
- Babits R., Szoke B., Sotonyi P. and Racz B. (2016) Food restriction modifies ultrastructure of hippocampal synapses. Hippocampus 26, 437–444. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

