Nonmonotonic recruitment of ventromedial prefrontal cortex during remote memory recall
- PMID: 29965966
- PMCID: PMC6044544
- DOI: 10.1371/journal.pbio.2005479
Nonmonotonic recruitment of ventromedial prefrontal cortex during remote memory recall
Abstract
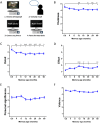
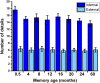
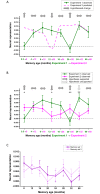
Systems-level consolidation refers to the time-dependent reorganisation of memory traces in the neocortex, a process in which the ventromedial prefrontal cortex (vmPFC) has been implicated. Capturing the precise temporal evolution of this crucial process in humans has long proved elusive. Here, we used multivariate methods and a longitudinal functional magnetic resonance imaging (fMRI) design to detect, with high granularity, the extent to which autobiographical memories of different ages were represented in vmPFC and how this changed over time. We observed an unexpected time course of vmPFC recruitment during retrieval, rising and falling around an initial peak of 8-12 months, before reengaging for older 2- and 5-year-old memories. This pattern was replicated in 2 independent sets of memories. Moreover, it was further replicated in a follow-up study 8 months later with the same participants and memories, for which the individual memory representations had undergone their hypothesised strengthening or weakening over time. We conclude that the temporal engagement of vmPFC in memory retrieval seems to be nonmonotonic, revealing a complex relationship between systems-level consolidation and prefrontal cortex recruitment that is unaccounted for by current theories.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

